
Dosimetric predictors of acute hematologic toxicity due to intensity-modulated pelvic radiotherapy with concurrent chemotherapy for pelvic cancer patients
Introduction
Chemoradiotherapy has improved the outcomes for pelvic cancer patients but at the cost of high levels of hematologic toxicity (HT). HT may be more significant for pelvic cancer patients because approximately one-third of adult bone marrow is located in the pelvic region (1-3). A significant number of cervical cancer patients undergoing chemoradiotherapy will experience increased HT compared with those undergoing radiotherapy (RT) alone, leading to an increased risk of infection, the use of granulocyte colony-stimulating factor, an extended treatment period, delayed adjuvant chemotherapy, and worse progression-free and overall survival (4-9). Reducing HT would also benefit patients with rectal cancer (10-14).
Several studies have demonstrated a correlation between pelvic bone marrow (PBM) dosimetric parameters and HT in patients with anal cancer and cervical cancer (15-25). Maintaining the mean PBM dose at <22.5 and <25 Gy is associated with a 5% and 10% risk of acute HT, respectively, in patients with anal cancer (16). Data from cervical cancer suggest that patients with PBM-V10 ≥90% have higher rates of grade 2 HT than patients with PBM-V10 <90% (21). However, the clinical significance and optimal PBM-sparing technique for rectal cancer patients remain unknown.
The purpose of this study was to identify potential dosimetric parameters that could predict HT in Chinese cervical and rectal cancer patients treated with pelvic intensity-modulated RT (IMRT) alone or in combination with concurrent chemotherapy.
Methods
Patients
We retrospectively analyzed 171 consecutive cervical and rectal cancer patients treated with pelvic IMRT alone or with concurrent chemoradiotherapy in the Second Affiliated Hospital of Soochow University between January 2015 and December 2016. Patients with and without surgery were included. The surgical procedures for cervical cancer included total abdominal hysterectomy and radical hysterectomy, and the surgical procedures for rectal cancer included the Miles’ operation, the Dixon operation, and local excision. The pathology results showed that the cervical cancers were squamous carcinomas or adenocarcinomas invading deep into the myometrium or parametrium with a diameter over 4 cm. Rectal cancer patients with stage T3–4 disease or pelvic lymphatic metastasis received preoperative or postoperative chemoradiotherapy.
IMRT simulation, planning, and delivery
A CT scan of each patient in the treatment position was obtained using our departmental scanner (AcQSim CT, Siemens Medical Systems, Germany) with a slice interval and thickness of 5 mm. Images were scanned from the L2 vertebral body to 5 cm below the ischial tuberosities and imported to the PINNACLE planning system (Philips Radiation Oncology Systems, Milpitas, CA, USA). For cervical cancer patients, the clinical target volume (CTV) consisted of the cervical tumor, the paracervical and parametrial tissues relative to the sidewall, the presacral region, the upper half of the vagina, the uterus, and the at-risk lymph nodes (common, internal, obturator, and external lymph nodes). Nodal regions were identified by adding a 7-mm margin around the contrast-enhanced vessels. For rectal cancer patients, CTV was defined as the gross tumor plus areas considered to be at significant risk of harboring microscopic disease, including the mesorectum, presacral region, and lateral lymph node region. For a select group of rectal patients, CTV also included the external iliac node region, ischiorectal fossa, sphincter complex, or inguinal node region. The planning target volume (PTV) was generated by adding a 7-mm margin around the CTV in the lateral and anterior-posterior directions and a 10-mm margin in the superior-inferior direction. Normal tissues included the bowel, bladder, rectum, and PBM.
The prescribed dose to the PTV was 45–50 Gy in 1.8-Gy or 2.0-Gy daily fractions. Cervical cancer patients without surgery received brachytherapy after IMRT. Some patients with locally advanced cervical cancer were treated with an integrated or sequential IMRT boost to the sites of gross disease. The IMRT plans were generated using the inverse planning module of PINNACLE for a 6-MV linear accelerator, with seven coplanar fields. The target goals specified that at least 95% of the PTV would receive the prescription dose, no more than 1% of the PTV receive >107% of the prescription dose, and no more than 1% of the PTV receive <93% of the prescription dose. The protocol specified that V45 of the small bowel was limited to 25%, V50 of the rectum was limited to 50%, and V50 of the bladder was limited to 40%. The dose constraints of bone marrow were that V10 be limited to 90% and that V20 be limited to 75%.
Bone marrow contouring and dose-volume histogram analysis
For all analyzed patients, the pelvic bone from the superior aspect to the inferior of the PTV was auto-contoured as a surrogate for PBM, and the autocontouring was accomplished with a CT density-based autocontouring algorithm by including tissue with a density of 1,000 to 4,000 HU on each slice throughout the whole PTV. The contour included the lumbosacral bone marrow; the iliac bone marrow; and the ischium, pubis, and proximal femoral bone marrow. This threshold was adjusted slightly for each patient to produce the most accurate contouring. The following dosimetric data were extracted from the dose-volume histograms (DVH): the relative volumes of PBM receiving 5, 10, 20, 30, 40, and 50 Gy (V5, V10, V20, V30, V40, and V50, respectively) and the mean doses of PBM.
Statistics
Acute HT was graded according to the Common Terminology Criteria for Adverse Events, version 3.0. Acute toxicity in this analysis was defined within 60 days after the start of radiotherapy. The highest-grade toxicity values for white blood count, absolute neutrophil count, hemoglobin, and platelets were recorded, with HT of grade ≥2 noted as an event. The χ2 test was used to test the correlation between the volume of bone marrow irradiation from 5 to 50 Gy (V5, V10, V20, V30, V40, and V50) and HT for patients treated with pelvic IMRT alone or together with chemoradiotherapy. In addition, univariate and multiple logistic regression analyses were performed to correlate the risk of grade ≥2 HT and the aforementioned predictors (i.e., age, sex, pathology, bone marrow irradiation volume, the number of chemotherapy cycles, cancer site and concurrent chemotherapy or consecutive chemotherapy). Statistical analysis was performed using SPSS software (version 19.0, SPSS Inc., USA). A value of P≤0.05 was considered statistically significant.
Results
Patients and treatment characteristics
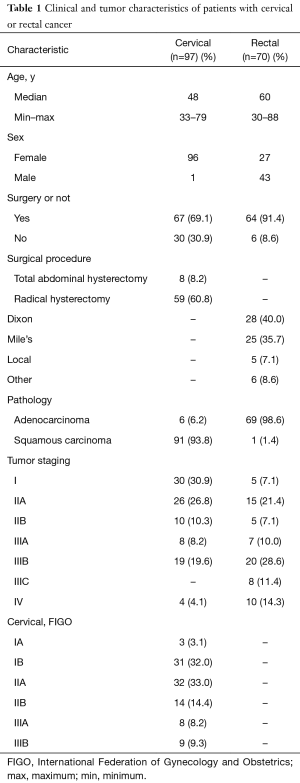
We retrospectively analyzed 171 patients, 5 patients did not complete treatment because of severe diarrhea. One hundred sixty-seven patients (97 with cervical cancer and 70 with rectal cancer) treated with pelvic IMRT were enrolled and analyzed finally. The mean ages were 48 and 60 years for cervical and rectal cancer, respectively. Table 1 summarizes the characteristics of the patients undergoing pelvic IMRT alone or with concurrent chemoradiotherapy.
Full table
Chemotherapy delivery
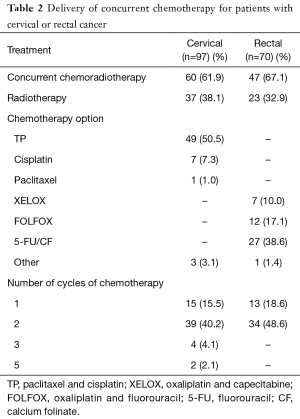
The cervical cancer patients received 2 cycles of paclitaxel (135–175 mg/m2) and cisplatin (30 mg/m2), while the rectal cancer patients received fluorouracil or its derivative during pelvic IMRT. The details for the delivery of concurrent chemotherapy are shown in Table 2. Sixty cervical cancer patients (61.9%) received concurrent chemoradiotherapy, among which 49 patients received 1 to 2 cycles of paclitaxel and cisplatin. Thirty-seven patients received radiotherapy alone. For rectal cancer patients, 47 (67.1%) received concurrent chemoradiotherapy, among which 39 patients received 1 to 2 cycles of fluorouracil or oxaliplatin plus fluorouracil. Twenty-three patients received radiotherapy alone.
Full table
Radiation delivery and dosimetric parameters
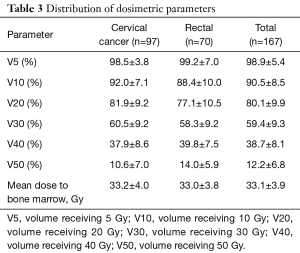
The mean total dose for PTV was 50.0 Gy (34.0–66.0 Gy). A few of cervical cancer patients received brachytherapy after IMRT. The descriptive statistics of the dose-volume parameters are listed in Table 3. The mean percentage volumes of bone marrow receiving 5, 10, 20, 30, 40 and 50 Gy for all patients were 98.9%, 90.5%, 80.1%, 59.4%, 38.7%, and 12.2%, respectively. In addition, the mean dose to the bone marrow was 33.1 Gy.
Full table
Hematologic toxicity
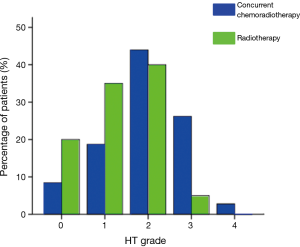
Among the 50 patients treated with pelvic IMRT alone, 21 (35%) experienced grade 1 HT, 24 (40%) experienced grade 2 HT, and 3 (5%) experienced grade 3 HT. Among the 107 patients treated with chemoradiotherapy, 20 (18.7%) experienced grade 1 HT, 47 (43.9%) experienced grade 2 HT, 28 (26.2%) experienced grade 3 HT, and 3 (2.8%) experienced grade 4 HT. The rates from grade 0 to 4 HT for patients treated with radiotherapy alone or in combination with chemoradiotherapy are shown in Figure 1.
Correlation between the irradiated bone marrow volume and HT
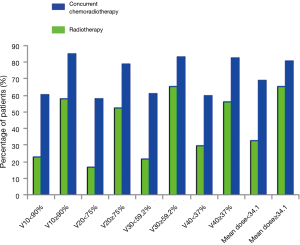
Among the patients treated with IMRT alone, an increased V10, V20, V30, V40, and mean PBM dose were significantly associated with increased HT during radiotherapy by χ2 test (57.9% vs. 22.7%, P=0.008; 52.1% vs. 16.7%, P=0.049; 65.6% vs. 21.4%, P=0.001; 56.3% vs. 29.6%, P=0.040; 65.2% vs. 32.4%, P=0.013; respectively). No statistically significant correlation was evident between V5 or V50 and HT. The patients receiving chemoradiotherapy with V10 ≥90%, V20 ≥75%, V30 ≥59.2%, and V40 ≥37% also had higher rates of grade 2 or higher HT than those with V10 <90%, V20 <75%, V30 <59.2%, and V40 <37% (85.2% vs. 60.4%, P=0.004; 78.9% vs. 58.1%, P=0.027; 83.3% vs. 60.8%, P=0.010; 82.3% vs. 60.0%, P=0.011; respectively) (Figure 2).
The univariate regression analysis showed that the following factors were significantly associated with increased HT for patients receiving concurrent chemoradiotherapy: cervical cancer (P=0.002, OR, 0.24), female sex (P=0.001, OR, 4.77), older than 60 years (P=0.009, OR, 0.28), squamous carcinoma (P=0.003, OR, 4.20), modality of concurrent chemotherapy (P=0.026, OR 0.84), and the number of cycles of concurrent chemotherapy (P=0.043, OR, 2.34). No statistically significant correlation was evident between HT and the number of cycles of consecutive chemotherapy. For patients treated with radiotherapy alone, none of the above factors were significantly associated with HT. The impacts of the factors on HT are shown in Table 4.
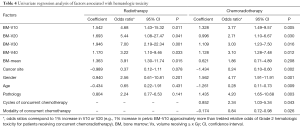
Full table
Multivariate logistic regression analysis of potential predictors showed that V10 and V40 (volume of whole pelvis PBM receiving 10 and 40 Gy, respectively) were significant for predicting HT in patients treated with chemoradiotherapy [odds ratio (OR), 4.07; 95% confidence interval (CI), 1.38–11.96; P=0.011; OR, 3.41; 95% CI, 1.20–9.69; P=0.022; respectively]. None of the other clinical parameters, including age, sex, pathology, number of chemotherapy cycles, cancer site, concurrent chemotherapy modality, and other dosimetric parameters, namely, the different dose levels (V5, V20, V30, V50, mean PBM dose) of the pelvis, were significant predictors of acute HT.
Discussion
Concurrent chemoradiotherapy is the standard treatment for women with locoregionally advanced cervical cancer. Preoperative chemoradiotherapy followed by total mesorectal excision is the standard of care for patients with locally advanced rectal cancer. However, an increase in the treatment effect can cause relevant side effects, especially HTs (4-9). HT remains a significant problem that can interrupt radiotherapy, limit chemotherapy delivery, necessitate transfusions and growth factors, and occasionally cause life-threatening infections. Numerous studies have demonstrated that, compared with conventional radiotherapy, pelvic IMRT can decrease the dose to normal tissues, such as the bladder, rectum, and bone marrow, and ameliorate relevant radiation-induced cystitis, enteritis and bone marrow suppression (26,27). At the same time, IMRT reduced the pelvic PBM volume that was irradiated by high doses compared with the AP-PA plans (22).
Numerous studies have demonstrated the high radiosensitivity of PBM stem cells (1). Because bone marrow stem cells are extremely sensitive to radiation, standard pelvic irradiation may damage a substantial amount of the bone marrow, resulting in the depletion of hematopoietic stem cells, which are vital to repopulating erythrocytes, leukocytes, and platelets (28). Therefore, limiting the bone marrow irradiation volume may attenuate the damage to bone marrow stem cells and alleviate HTs.
How can we constrain the bone marrow irradiation volume? Mell has reported that the V10 and V20 of the low-dose volumes are associated with acute HT in cervical and anal cancer patients receiving concurrent chemoradiotherapy, whereas the RTOG 0418 study of gynecologic cancer has shown that V40 is the best predictor of acute HT in concurrent pelvic chemoradiotherapy (21-23). The reason for this difference may be the insufficient protection of the bone marrow in the RTOG 0418 study and the few cases of V10 less than 90% (n=12). Several current studies have undertaken bone marrow-sparing IMRT for cervical cancer patients, but few studies have examined bone marrow-sparing IMRT for rectal cancer patients. Hence the objective of this study was to explore the benefits of bone marrow-sparing IMRT and ascertain the quantified dosimetric predictors for HTs. In addition, this study quantitatively showed that the dose constraint for bone marrow in IMRT ameliorated acute HT in cervical and rectal cancer patients.
The novelty of this study was to evaluate Chinese people. Our study showed that acute HT was associated with V10, V20, V30, V40 and the mean PBM dose, which was consistent with previous studies. High volume of PBM receiving irradiation can interrupt radiotherapy, limit chemotherapy delivery, and finally, suspend treatment. However, we did not find that V20 correlated with acute HT in patients treated with concurrent chemoradiotherapy. This observation may have resulted from the diversified chemotherapy modalities used for the cervical and rectal cancer patients and from the application of granulocyte colony-stimulating factors. In addition, because chemotherapy can lead to acute blood toxicity in the same way, the advantage of bone marrow-sparing IMRT to reduce acute HT may be offset by chemotherapy. At last, our research is aimed at the Chinese, which may lead to slight differences with previous studies.
Despite being retrospective, this study provides a meaningful reference for future studies. We also investigated the effect of radiotherapy alone to exclude the interference of chemotherapy in acute HT, and the result demonstrated the contribution of radiotherapy to acute HT. In the univariate analysis, the bone marrow irradiation volume associated with HT was the same for radiotherapy alone and concurrent chemoradiotherapy. This indicated that the limitation of PBM dose could reduce HT, whether it was radiotherapy alone or concurrent chemoradiotherapy.
CT images are widely used currently for localization of precise radiotherapy. Pelvic bones are recognized as a surrogate for bone marrow in the irradiation volume for dose constraint, which could be overestimated for bone marrow and result in deviations in the dose evaluation for bone marrow (23). Normal bone marrow is divided into the red bone marrow and the yellow bone marrow. Red bone marrow is responsible for hematopoiesis, but yellow bone marrow not. The two types of bone marrow cannot be distinguished in CT images. However, magnetic resonance imaging (MRI), which is extremely sensitive to fat and water, can enable the distinction (17,25,29). Imaging techniques, such as single photon emission computed tomography (SPECT) and MRI, have been proposed to optimize PBM-sparing IMRT plans by identifying hematopoietically active regions of PBM that can be spared preferentially. Future explorations should emphasize an appropriate imaging modality to delineate PBM to more precisely evaluate the bone marrow irradiation volume and recognize more efficient predictors of acute HT.
No recommendation is provided by the Quantitative Analysis of Normal Tissue Effects in the Clinic (QUANTEC) regarding bone marrow dose constraints. However, we strongly suggest that PBM be routinely included as an organ at risk (OAR) for cervical and rectal cancer patients receiving pelvic radiotherapy alone or with concurrent chemoradiotherapy.
Limitations
This study had several limitations due to the retrospective nature of the study, namely, the small sample size and small number of female patients.
Conclusions
This study lends strong support to the hypothesis that V10 and V40 of PBM are important predictors of HT both in cervical and rectal cancer patients undergoing intensity-modulated pelvic radiotherapy with concurrent chemotherapy. Efforts to maintain BM-V10 ≤90% and BM-V40 ≤37% can significantly reduce grade ≥2 HT. These results suggest the potential to optimize bone marrow-sparing IMRT plans to reduce the toxicity of chemoradiotherapy and possibly improve treatment outcomes by allowing better chemotherapy delivery. Future research on bone marrow-sparing IMRT is needed, including prospective studies evaluating its efficacy.
Acknowledgments
Funding: This work was supported by Suzhou “Revitalizing Healthcare with Science and Education” Youth Science and Technology Project (No. KJXW2016010); Jiangsu Medical Innovation Team (No. CXDT-37); Suzhou Science and Technology Development Program (No. SZS201509); Suzhou Clinical Medical Center Construction Project (No. Szzxj201503); Medicine Outstanding Leader of Suzhou (No. 62).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.05.04). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The written informed consent was waived due to the retrospective nature of the study. The study was approved by the ethics committee of The Second Affiliated Hospital of Soochow University (No. 2017045).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mauch P, Constine L, Greenberger J, et al. Hematopoietic stem cell compartment: acute and late effects of radiation therapy and chemotherapy. Int J Radiat Oncol Biol Phys 1995;31:1319-39. [Crossref] [PubMed]
- Blebea JS, Houseni M, Torigian DA, et al. Structural and functional imaging of normal bone marrow and evaluation of its age-related changes. Semin Nucl Med 2007;37:185-94. [Crossref] [PubMed]
- Hayman JA, Callahan JW, Herschtal A, et al. Distribution of proliferating bone marrow in adult cancer patients determined using FLT-PET imaging. Int J Radiat Oncol Biol Phys 2011;79:847-52. [Crossref] [PubMed]
- Ohno T, Kato S, Wakatsuki M, et al. Incidence and temporal pattern of anorexia, diarrhea, weight loss, and leukopenia in patients with cervical cancer treated with concurrent radiation therapy and weekly cisplatin: comparison with radiation therapy alone. Gynecol Oncol 2006;103:94-9. [Crossref] [PubMed]
- Dueñas-González A, Zarba JJ, Patel F, et al. Phase III, open-label, randomized study comparing concurrent gemcitabine plus cisplatin and radiation followed by adjuvant gemcitabine and cisplatin versus concurrent cisplatin and radiation in patients with stage IIB to IVA carcinoma of the cervix. J Clin Oncol 2011;29:1678-85. [Crossref] [PubMed]
- Torres MA, Jhingran A, Thames HD Jr, et al. Comparison of treatment tolerance and outcomes in patients with cervical cancer treated with concurrent chemoradiotherapy in a prospective randomized trial or with standard treatment. Int J Radiat Oncol Biol Phys 2008;70:118-25. [Crossref] [PubMed]
- Nugent EK, Case AS, Hoff JT, et al. Chemoradiation in locally advanced cervical carcinoma: An analysis of cisplatin dosing and other clinical prognostic factors. Gynecol Oncol 2010;116:438-41. [Crossref] [PubMed]
- Parker K, Gallop-Evans E, Hanna L, et al. Five years’ experience treating locally advanced cervical cancer with concurrent chemoradiotherapy and high-dose-rate brachytherapy: Results from a single institution. Int J Radiat Oncol Biol Phys 2009;74:140-6. [Crossref] [PubMed]
- Chen SW, Liang JA, Hung YC, et al. Concurrent weekly cisplatin plus external beam radiotherapy and high-dose rate brachytherapy for advanced cervical cancer: A control cohort comparison with radiation alone on treatment outcome and complications. Int J Radiat Oncol Biol Phys 2006;66:1370-7. [Crossref] [PubMed]
- Yang TJ, Oh JH, Apte A, et al. Clinical and dosimetric predictors of acute hematologic toxicity in rectal cancer patients undergoing chemoradiotherapy. Radiother Oncol 2014;113:29-34. [Crossref] [PubMed]
- Wan J, Liu K, Li K, et al. Can dosimetric parameters predict acute hematologic toxicity in rectal cancer patients treated with intensity-modulated pelvic radiotherapy? Radiat Oncol 2015;10:162. [Crossref] [PubMed]
- Jianyang W, Yuan T, Yuan T, et al. A prospective phase II study of magnetic resonance imaging guided hematopoietical bone marrow-sparing intensity-modulated radiotherapy with concurrent chemotherapy for rectal cancer. Radiol Med 2016;121:308-14. [Crossref] [PubMed]
- Newman NB, Sidhu MK, Baby R, et al. Long-Term Bone Marrow Suppression During Postoperative Chemotherapy in Rectal Cancer Patients After Preoperative Chemoradiation Therapy. Int J Radiat Oncol Biol Phys 2016;94:1052-60. [Crossref] [PubMed]
- Newman NB, Moss RA, Maloney-Patel N, et al. Bone marrow tolerance during postoperative chemotherapy in colorectal carcinomas. J Gastrointest Oncol 2017;8:547-555. [Crossref] [PubMed]
- Mell LK, Schomas DA, Salama JK, et al. Association between bone marrow dosimetric parameters and acute hematologic toxicity in anal cancer patients treated with concurrent chemotherapy and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 2008;70:1431-7. [Crossref] [PubMed]
- Bazan JG, Luxton G, Mok EC, et al. Normal tissue complication probability modeling of acute hematological toxicity in patients treated with intensity-modulated radiation therapy for squamous cell carcinoma of the anal canal. Int J Radiat Oncol Biol Phys 2012;84:700-6. [Crossref] [PubMed]
- Franco P, Arcadipane F, Ragona R, et al. Dose to specific subregions of pelvic bone marrow defined with FDG-PET as a predictor of hematologic nadirs during concomitant chemoradiation in anal cancer patients. Med Oncol 2016;33:72. [Crossref] [PubMed]
- Julie DA, Oh JH, Apte AP, et al. Predictors of acute toxicities during definitive chemoradiation using intensity-modulated radiotherapy for anal squamous cell carcinoma. Acta Oncol 2016;55:208-16. [Crossref] [PubMed]
- Franco P, Ragona R, Arcadipane F, et al. Dosimetric predictors of acute hematologic toxicity during concurrent intensity-modulated radiotherapy and chemotherapy for anal cancer. Clin Transl Oncol 2017;19:67-75. [Crossref] [PubMed]
- Lee AY, Golden DW, Bazan JG, et al. Hematologic Nadirs During Chemoradiation for Anal Cancer: Temporal Characterization and Dosimetric Predictors. Int J Radiat Oncol Biol Phys 2017;97:306-12. [Crossref] [PubMed]
- Mell LK, Kochanski JD, Roeske JC, et al. Dosimetric predictors of acute hematologic toxicity in cervical cancer patients with concurrent cisplatin and intensity-modulated pelvic radiotherapy. Int J Radiat Oncol Biol Phys 2006;66:1356-65. [Crossref] [PubMed]
- Rose BS, Aydogan B, Liang Y, et al. Normal tissue complication probability modeling of acute hematologic toxicity in cervical cancer patients treated with chemoradiotherapy. Int J Radiat Oncol Biol Phys 2011;79:800-7. [Crossref] [PubMed]
- Klopp AH, Moughan J, Portelance L, et al. Hematologic toxicity in RTOG 0418: a phase 2 study of postoperative IMRT for gynecologic cancer. Int J Radiat Oncol Biol Phys 2013;86:83-90. [Crossref] [PubMed]
- Noticewala SS, Li N, Williamson CW, et al. Longitudinal changes in active bone marrow for cervical cancer patients treated with concurrent chemoradiation therapy. Int J Radiat Oncol Biol Phys 2017;97:797-805. [Crossref] [PubMed]
- Li N, Noticewala SS, Williamson CW, et al. Feasibility of atlas-based active bone marrow sparing intensity modulated radiation therapy for cervical cancer. Radiother Oncol 2017;123:325-30. [Crossref] [PubMed]
- Brixey CJ, Roeske JC, Lujan AE, et al. Impact of intensity-modulated radiotherapy on acute hematologic toxicity in women with gynecologic malignancies. Int J Radiat Oncol Biol Phys 2002;54:1388-96. [Crossref] [PubMed]
- Mundt AJ, Lujan A, Rotmensch J, et al. Intensity-modulated whole pelvic radiotherapy in women with gynecologic malignancies. Int J Radiat Oncol Biol Phys 2002;52:1330-7. [Crossref] [PubMed]
- Cao X, Wu X, Frassica D, et al. Irradiation induces bone injury by damaging bone marrow microenvironment for stem cells. Proc Natl Acad Sci U S A 2011;108:1609-14. [Crossref] [PubMed]
- Rose BS, Liang Y, Lau SK, et al. Correlation between radiation dose to 18F-FDG-PET defined active bone marrow subregions and acute hematologic toxicity in cervical cancer patients treated with chemoradiotherapy. Int J Radiat Oncol Biol Phys 2012;83:1185-91. [Crossref] [PubMed]


