
A novel approach to cancer therapy using melanin-containing patches
In recent decades, the incidence of melanoma has been steadily raising, even if it is still considered to be a rare cancer. In contrast with what is observed in other solid tumors, melanomas principally affect young and middle-aged individuals who are often still in the prime of their carriers and are involved in raising a family. Surgery is the first-line treatment, in particular for early-stage melanomas; unfortunately, patients often develop metastasis, and the presence of a first tumor increases the chance to develop a second melanoma (1).
Immunotherapy is one of most promising approach for the treatment of this kind of tumor (in addition to targeted-, chemo- and radio-therapy). Principally, it is based on the use of immunomodulatory cytokines (2) or of checkpoint inhibitors (proteins present on immune cells that can switch on or off the cascade of events and processes that culminate in the immune response) (3). Candidate immunotherapy protocols also envision the administration of ex vivo activated immune cells, such as dendritic cells (DCs) or T lymphocytes (4). Immunotherapeutic approaches were shown to be promising; this premise notwithstanding, the efficacy and feasibility of this type of therapy need to be improved.
In this context, the paper entitled “A melanin-mediated cancer immunotherapy patch” analyzes the use of intradermal injection of melanin and tumor antigens, in combination with near-infrared light irradiation, as a therapeutic vaccine approach finalized at activating and improving the specific immune response against melanoma (5). The strategy reported in the paper is based on the filling of a silicone mold with an array of 15×15 microneedles (MN) with GM-CSF (granulocyte-macrophage colony-stimulating factor) in the presence of whole melanoma tumor lysate (Figure 1).
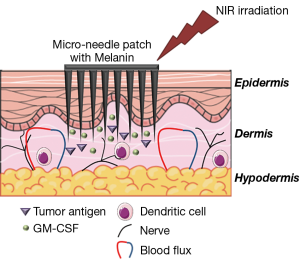
The release of GM-CSF after dermal positioning of the patch facilitates the uptake of the tumor antigens contained in the lysate and their presentation by DCs, the professional antigen presenting cells (APCs) principally involved in anti-tumor immune responses (6). GM-CSF release and the homing of activated DC in lymph nodes are fundamental steps in the activation of anti-tumoral immune responses. These processes are impaired in patients and are not corrected by cell-based immunotherapies, leading to the unsatisfactory results that still beset these therapies (7). Notably, since melanoma is due to the deregulated growth of melanocytes, tumor lysate contains high level of melanin (8), a natural pigment of the skin that converts the light energy in heat. The presence of melanin induces a darkening of the patch, increasing the absorption of light, when it is irradiated with near infra-red (NIR) light (at 800 nm). This results in an increase of the local temperatures (up to 42 °C), leading to a better presentation of antigens as an effect of the activation of heat shock proteins. This protocol also enhances the immunogenicity of tumor lysate, that form HSP-antigen complexes with MHC-class I molecules (9), and stimulates the release of inflammatory cytokines, which in turn amplify the recruitment of other immune cells (10). Finally, the mild increase of local temperature contributes to heighten the local blood and lymphatic fluxes, facilitating again the migration of DCs, T cells, and other immune cells, such as natural killer cells (NK) (11).
This MN-based immunotherapy was tested on mice. On day 0, GM-CSF and tumor lysate loaded MN patches were applied on the skin of healthy mice, and half of them underwent NIR irradiation for 5 consecutive days. After 10 days, mice were subcutaneously inoculated with the B16F10 melanoma cell line. Mice receiving MNs without NIR irradiation showed 13% of protection respect to control group (that were sacrificed within 25 days), whereas mice that receive combined treatment (MN + NIR) showed long-term protection, with complete tumor rejection in 87% of cases. Notably, a 6-fold increase in the number of DC present in the treated skin section, a 10-fold increase of CD8+ T cells, an 8-fold increase of Ig titers, and prolonged immune response, were also observed in the MN + NIR mice. Very importantly, the immune response observed in the MN + NIR animals prevented the growth of tumor masses located far from the MN patch; conferred resistance to a secondary tumor challenge; and prevented the formation of metastases, suggesting that this protocol results on the elicitation of potent and persistent systemic immune responses. The specificity of the immune response that was stimulated in MN + NIR mice was confirmed by the observation that when these mice were inoculated with a different (non-melanoma) tumor, no protective effects were observed.
Previously, the same group tested in mice a MN patch for the delivery of programmed death-1 (PD1)-specific antibodies. Also in this case, the use of MN for intradermal administration of the drug resulted in a higher survival time and was associated with an increase of CD4 and CD8 tumor-infiltrating lymphocytes into the tumor mass (12). Taken together these results seem to suggest that the use of MNs could be considered to be a simple and fast technique to increase cancer immunogenicity.
In fact, transdermal drug delivery is endowed with noticeable potential advantages, as this methodology allows the drug to be released locally, thus greatly reducing side effects, as well as eliminating pre-systemic first-pass effect (Figure 1). A number of problems nevertheless need to be addressed including the type and dose of therapeutics that can be delivered through transdermal administration, skin irritation and biocompatibility of used materials, microbial contamination, mechanical strength and accidental use and abuse due to the apparent safety of the patch (13,14).
MNs were principally tested in the clinical setting for vaccine delivery (NCT02621112 HBV, NCT01518478 Fluzone®, NCT01813604 anti-polio, NCT00558649 FLUARIX®). Since vaccines are delivered by the intradermal/intramuscular route, this seems to be the natural evolution of this kind of administration. Notably, results obtained within this setting showed that a strong activation of adaptive immunity and, in particular, of T lymphocytes (thanks to the activation of NF-kB-driven inflammation, IFNγ response and TNF and CD40 signaling) can be achieved by using MNs (15,16).
In oncology, promising results were generated in a recent clinical trial in patients suffering from actinic keratosis (NCT02594644) (17,18), a pre-cancerous lesion of skin that could develop into a skin cancer. Thus, the application of a MN patch containing Aminolevulinic acid (ALA) followed by blue light irradiation for 20 minutes resulted in actinic keratosis clearance in 76% of cases, with 3 patients achieving complete remission. The clearance rate was similar to that observed in the conventional 1-hour ALA and irradiation treatment, but use of the MN patch was associated with a faster time therapeutic protocol, a higher absorption of ALA and a strong reduction of pain during light exposure. Notably, a phase I-trial is currently ongoing for the delivery of doxorubicin through MNs for the treatment of ccutaneous T-cell lymphoma (NCT02192021) (18) to evaluate the therapeutic dose, and its efficacy and safety.
The use of melanin to generate heat to promote antigen uptake in the context of intradermal injection has been analyzed in other experimental models, and in particular in tumors that do not contain melanin (e.g., triple negative breast cancer, including tumors derived from 4T1 cell line); in this case synthetic melanin was loaded into the patches. Also in this setting, a positive hyperthermic effect following irradiation with NIR light was observed (5). In fact, authors reported that vaccination with MNs and NIR irradiation resulted in an 87% rejection rate of 4T1 tumors, with 37% of complete remission being detected in mice undergoing combined therapy. Notably, this was associated with a 4-fold increase of HSP70, higher levels of pro-inflammatory cytokines and DC activation (5).
Melanin is characterized by a broad spectrum of light absorption, that is located in the ultraviolet and visible range. Normally, the pigments present in the skin, such as carotene and hemoglobin, are characterized by a clear peak of absorption, whereas melanin shows an unusual broadband spectrum of absorption (Figure 2): that decreases in a quite linear way towards higher wavelengths, characterized by photon with low energy. Probably, this is due to the photo-protective role of melanin against UV and light damage: in fact, a spectrum characterized by a well-defined peak of absorption allows photons of other wavelengths to be transmitted, with possible consequent cell damage. These peculiarities allow the use a huge range of wavelengths to stimulate melanin. The authors used light in the NIR window, probably due to the low energy of these photons which do not induce cell damage. Notably, the fine-tuning of this approach will likely allow in the near future, the guided-release of drugs loaded in the MN patch (19,20).
In conclusion, the study published by Ye et al. show that, in the animal model, patches with microneedles for transdermal delivery of tumor antigens and cytokines can be used to optimally trigger tumor-specific immune responses as an effect of their ability to potently activate DC. Interestingly, the use of Melanin and NIR irradiation further increased the potency of immune responses via the activation of heat-shock protein and an enhanced recruitment of immune cells due to heat-driven increase of blood flux. These initial promising results pave the way for the development of more efficient immune-based therapy for the management of oncologic disease.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Chen Qian (Center for Inflammation & Epigenetics, Houston Methodist Hospital Research Institute, Houston, USA).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.05.23). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rastrelli M, Tropea S, Rossi CR, et al. Melanoma: Epidemiology, Risk Factors, Pathogenesis, Diagnosis and Classification. In Vivo 2014;28:1005-11. [PubMed]
- Xu DH, Zhu Z, Xiao H, et al. Unveil the mysterious mask of cytokine-based immunotherapy for melanoma. Cancer Lett 2017;394:43-51. [Crossref] [PubMed]
- Rodríguez-Cerdeira C, Carnero Gregorio M, López-Barcenas A, et al. Advances in Immunotherapy for Melanoma: A Comprehensive Review. Mediators Inflamm 2017;2017:3264217 [PubMed]
- de Vries IJ, Lesterhuis WJ, Barentsz JO, et al. Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat Biotechnol 2005;23:1407-13. [Crossref] [PubMed]
- Ye Y, Wang C, Zhang X, et al. A melanin-mediated cancer immunotherapy patch. Sci Immunol 2017;2: [Crossref] [PubMed]
- Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol 1997;9:10-6. [Crossref] [PubMed]
- Kim JV, Latouche JB, Riviére I, et al. The ABCs of artificial antigen presentation. Nat Biotechnol 2004;22:403-10. [Crossref] [PubMed]
- Brenner M, Hearing VJ. The Protective Role of Melanin Against UV Damage in Human Skin. Photochem Photobiol 2008;84:539-49. [Crossref] [PubMed]
- Qiu J, Li GW, Sui YF, et al. Heat-shocked tumor cell lysate pulsed dendritic cells induce effective anti-tumor immune response in vivo. World J Gastroenterol 2006;12:473-8. [Crossref] [PubMed]
- Borish LC, Steinke JW. 2. Cytokines and chemokines. J Allergy Clin Immunol 2003;111:S460-75. [Crossref] [PubMed]
- Evans SS, Repasky EA, Fisher DT. Fever and the thermal regulation of immunity: the immune system feels the heat. Nat Rev Immunol 2015;15:335-49. [Crossref] [PubMed]
- Wang C, Ye Y, Hochu GM, et al. Enhanced Cancer Immunotherapy by Microneedle patch-assisted Delivery of anti-PD1 Antibody. Nano Lett. 2016;16:2334-40. [Crossref] [PubMed]
- Ita K. Transdermal Delivery of Drugs with Microneedles—Potential and Challenges. Pharmaceutics 2015;7:90-105. [Crossref] [PubMed]
- Birchall JC, Clemo R, Anstey A, et al. Microneedles in clinical practice--an exploratory study into the opinions of healthcare professionals and the public. Pharm Res 2011;28:95-106. [Crossref] [PubMed]
- Kis EE, Winter G, Myschik J. Devices for intradermal vaccination. Vaccine 2012;30:523-38. [Crossref] [PubMed]
- Bragazzi NL, Orsi A, Ansaldi F, et al. Fluzone® intra-dermal (Intanza®/Istivac® Intra-dermal): An updated overview. Hum Vaccin Immunother 2016;12:2616-27. [Crossref] [PubMed]
- Petukhova TA, Hassoun LA, Foolad N, et al. Effect of Expedited Microneedle-Assisted Photodynamic Therapy for Field Treatment of Actinic Keratoses. A Randomized Clinical Trial. JAMA Dermatol 2017;153:637-43. [Crossref] [PubMed]
- Bhatnagar S, Dave K, Venuganti VV. Microneedles in the clinic. J Control Release 2017;260:164-82. [Crossref] [PubMed]
- Xia B, Wang B, Chen Z, et al. Near-Infrared Light-Triggered Intracellular Delivery of Anticancer Drugs Using Porous Silicon Nanoparticles Conjugated with IR820 Dyes. Adv Mater Interfaces 2016;3:1500715 [Crossref]
- Lim DJ, Park H. Near-infrared light for on-demand drug delivery. J Biomater Sci Polym Ed 2018;29:750-61. [Crossref] [PubMed]



