
Efficacy, safety and prognostic factors analysis of first-line icotinib treatment in advanced non-small cell lung cancer patients with mutated EGFR
Introduction
Lung cancer is the most frequently diagnosed cancer and the most common cause of cancer-related deaths, which seriously threatens human health and life (1). According to 2015 cancer statistics, estimated 733,000 new cases and 610,000 deaths due to lung cancer occurred in China (2). Lung cancer is mainly classified into two subtypes, including small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC), which account for approximately 15% and 85% of all lung cancer respectively (3). Among these, more than 65% patients with NSCLC at the first diagnosis are in advanced stage or with metastatic disease, and the 5-year overall survival (OS) rate is only 15% (4). For these patients, conventional chemotherapy is one of the most frequently used treatments and could provide a modest survival advantage, while its response rate among these advanced NSCLC patients is still less than 40%, suggesting that the efficacy of conventional chemotherapy might reach the therapeutic plateau (5-7).
Epidermal growth factor receptor tyrosine kinase inhibitor (EGFR TKI) is recognized as the first-line therapy for NSCLC patients with active sensitizing EGFR mutation due to its superior efficacy over than conventional chemotherapy (8,9). Currently, gefitinib, erlotinib and afatinib, as three common EGFR TKIs, have established their values in NSCLC patients with EGFR-sensitive mutation, particularly exon 19 deletions and exon 21 L858R mutations (10,11). Icotinib, acting as another novel, orally administered, reversible small-molecule EGFR-TKI, is designed and patented by Beta Pharma (Zhejiang, China), which is approved by the State Food and Drug Administration of China for advanced NSCLC patients with EGFR mutation based on phase III of ICOGEN trial (12,13). Several previous studies have proven that icotinib contributes to good efficacy in advanced NSCLC patients as second-line treatment (14). Although there are several retrospective studies in China, while the sample sizes in most studies are relatively small, which are less than 50 cases, and few study with larger samples to confirm the effects of icotinib as first-line treatment in advanced NSCLC patients has been found (5,15). In the current study, we recruited 152 advanced NSCLC patients with EGFR mutation, and the purpose was to evaluate the efficacy, safety and prognostic factors of icotinib as first-line treatment in advanced NSCLC patients with EGFR-sensitive mutation.
Methods
Patients
A total of 152 advanced NSCLC patients with EGFR-sensitive mutation underwent first-line icotinib treatment at Shanghai Chest Hospital, Shanghai Jiao Tong University between November 2011 to November 2016 were retrospectively reviewed in this cohort study. The inclusion criteria were: (I) confirmed diagnosis of NSCLC with EGFR mutation by cytopathology and histopathology analysis; (II) patients received first-line icotinib treatment (patients did not receive surgery, chemotherapy, radiotherapy or any other treatments before icotinib administration); (III) patients were in stage IIIb and stage IV, who could not undergo surgery; (IV) more than one measurable lesion based on Response Evaluation Criteria in Solid Tumors 1.1 (RECIST, version 1.1) (16). The exclusion criteria were: (I) secondary lung cancer patients; (II) previous lung severe infection or transplantation; (III) complicated with severe underlying cardiopulmonary diseases, a history of interstitial lung disease or severe gastrointestinal disorders influencing drug absorption; (IV) lacked one of the following basic data including age, gender, smoking history, EGFR mutation type, TNM stage, brain metastasis status, treatment response evaluation.
This study was approved by Ethics Committee of Shanghai Chest Hospital, Shanghai Jiao Tong University. The number of ethical approval was KS1034. The written informed consents or oral agreements (with recording) were required from all patients or their guardians.
Detection of EGFR mutation
Scorpion amplification refractory mutation system (ARMS) kit (Qiagen, Venlo, the Netherlands) was used for EGFR mutation detection. EGFR sensitive mutations was defined as exon 19 deletions and exon 21 L858R mutations.
Treatment
Icotinib was given orally three times daily at 125 mg dose until tumor progression or intolerable drug toxicity, with no other systematic anticancer treatments such as chemotherapy and anticancer herbal therapy. If PD occurred, patients could further receive repeated icotinib treatment (if it was beneficial after evaluation), patients who were younger than 75 years old and with generally good conditions would further receive chemotherapy and patients who were older than 75 years old or with poor conditions (performance status score ≥2) would further received optimum supportive treatment.
Treatment response and adverse events (AEs) assessments
Baseline evaluation of all patients was completed within 1 week before treatment. The assessment included chest computed tomography (CT) scan, abdominal CT scan, Brain CT scan or magnetic resonance imaging (MRI) of the brain and whole body bone scan. RECIST criteria (version 1.1) was used to assess the treatment response as complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). The objective response rate (ORR) was defined as CR plus PR. The radiological evaluation was performed by two independent oncologists and when controversial results existed, a third oncologist was invited to vote. Generally, the treatment response was evaluated at 1–2 months after icotinib treatment. AEs were assessed according to the National Cancer Institute (NCI)-Common Terminology Criteria for Adverse Events (CTCAE) version 3.0.
Follow ups
Patients were followed up 1–2 months after icotinib treatment, then regularly followed up according to patients’ willing and disease condition. Due to medicine controls in China, every patient could be issued with 4-week dose of icotinib, thus, these NSCLC patients would come to Outpatient Clinic of the hospital to get more icotinib every 4 weeks. At this time, each patient would receive blood test, liver and kidney function examinations as well as tumor markers examinations. Meanwhile, imaging examination (CT scan) was performed every 8 weeks. The median follow-up duration was 15.0 (range: 1.0–44.0) months, and the last follow-up date was 2016/12/31. Progression-free survival (PFS) was calculated from the time of icotinib administration to documented progression or death from any cause. OS was calculated from the time of icotinib administration until the date of death from any causes.
Statistics
Statistics was performed using SPSS 24.0 (SPSS Inc., USA) and 2010 office software (Microsoft, USA). Data was presented as mean ± standard deviation or count (%). Kaplan-Meier (K-M) curves were preformed to assess PFS and OS after first-line icotinib treatment. Factors affecting ORR achievement were determined by univariate logistic regression analysis, while all factors with P value no more than 0.1 were further detected by multivariate logistic regression analysis. Factors affecting PFS and OS were determined by univariate Cox’s analysis. P value <0.05 was considered significant.
Results
Baseline characteristics
As listed in Table 1, mean age of 152 advanced NSCLC patients with EGFR mutation was 63.35±11.29 years. Males and females were 62 and 90 respectively. There were 48 (31.6%) patients with smoking history. As to EGFR mutation type, the numbers of patients with 19del and 21L858R were 84 (55.3%) and 68 (44.7%) respectively. Twenty-three (15.1%) patients were in stage IIIb and 129 (84.9%) patients were in stage IV. Twenty-eight (18.4%) patients were complicated with brain metastasis.
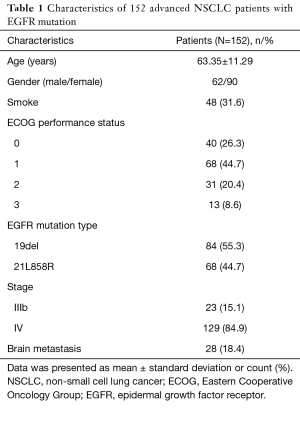
Full table
Treatment response after first-line icotinib treatment
After first-line icotinib treatment, the rates of CR and PR were 4.0% (N=6), 67.8% (N=103), and the rate of ORR, which was the combination of CR and PR, was 71.8% (N=109). In addition, the rates of SD and PD rates were 23.7% (N=36) and 4.6% (N=7) respectively (Table 2).
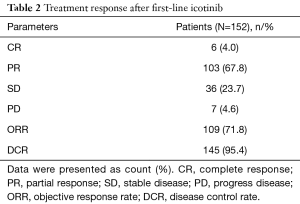
Full table
PFS and OS after first-line icotinib treatment
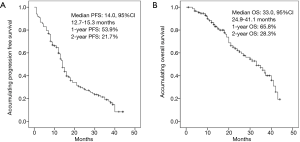
Median PFS was 14.0 months (95% CI: 12.7–15.3 months), and the rates of 1-year and 2-year PFS were 53.9% and 21.7% respectively (Figure 1A). As to OS, median OS was 33.0 months (95% CI: 24.9–41.1 months), and the rates of 1-year and 2-year OS were 65.8% and 28.3% respectively (Figure 1B).
Factors influencing ORR achievement
Factors affecting ORR achievement were assessed by logistic regression model analysis, which suggested that smoking history (P=0.005) was associated with a lower possibility for ORR achievement, while EGFR mutation 19del (P=0.001) were correlated with a higher possibility for ORR achievement (Table 3). All factors with a P value ≤0.1 in univariate logistic model were further analyzed by multivariate logistic regression model, which indicated smoking history (P=0.013) was statistically correlated with non-ORR achievement, while EGFR mutation 19del (P=0.001) was statistically associated with ORR achievement in advanced NSCLC patients with EGFR mutation after first-line icotinib treatment.
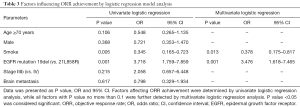
Full table
Factors influencing PFS
Univariate Cox’s proportional hazards regression model analysis was performed to evaluate factors influencing PFS, as presented in Table 4, which revealed that no factor (all P>0.05) could predict PFS in advanced NSCLC patients with EGFR mutation after first-line icotinib treatment. Due to all factors with P>0.1 in univariate Cox’s proportional hazards regression model analysis, multivariate Cox’s proportional hazards regression analysis was not performed.
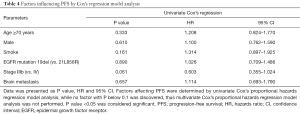
Full table
Factors influencing OS
Factors affecting OS was determined by univariate Cox’s proportional hazards regression model analysis (Table 5). No factor (All P>0.05) could be predictive factor for OS in advanced NSCLC patients with EGFR mutation after first-line icotinib treatment. Owning to no factor with P<0.1 in univariate Cox’s proportional hazards regression model analysis, multivariate Cox’s proportional hazards regression analysis was not performed.
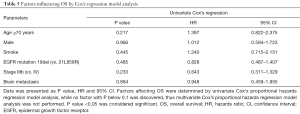
Full table
Safety profiles by first-line icotinib treatment
There were 66 (43.4%), 35 (23.0%), 10 (6.6%), 9 (5.9%), 9 (5.9%), 4 (2.6%) and 3 (2.0%) patients appearing rash, diarrhea, abnormal liver function, paronychia, oral ulcer, fatigue and poor appetite (Table 6). Most adverse events were of grade 1, except for 2 (1.3%) cases of grade 3 rash, and 2 (1.3%) cases of grade 2 diarrhea.
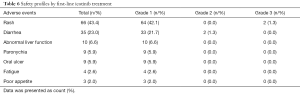
Full table
Correlation of metastasis types with treatment response
In the present study, there were 29 patients with CNS metastasis, and 1 case achieved CR and 19 cases achieved ORR. In addition, we further performed chi-square test to compare the CR rate and ORR rate between brain metastasis patients and others, and found there was no difference in CR rate (P=0.910) and ORR rate (P=0.616) between brain metastasis patients and others (Table 7).

Full table
Discussion
In the present study, we observed that: (I) first-line icotinib treatment achieved CR of 4% and ORR of 71.8% respectively in advanced NSCLC patients with EGFR-sensitive mutation received first-line icotinib treatment. Median PFS of 14.0 months (95% CI: 12.7–15.3 months) and OS of 33.0 months (95% CI: 24.9–41.1 months) were observed. (II) Smoking history was statistically correlated with non-ORR achievement, while EGFR mutation 19del (P=0.001) was statistically associated with ORR achievement. No factor affecting PFS and OS was observed. (III) After first-line icotinib treatment, rash, diarrhea and abnormal liver function were common AEs, and most AEs were in mild grade, suggesting that first-line icotinib treatment was well tolerated.
NSCLC is one of most complicated malignancies, and more than 50% NSCLC patients in China have been identified with EGFR mutation, which is one of multi-function transmembrane glycoproteins and involves in the process of cell proliferation, differentiation, migration, invasiveness, angiogenesis and adhesiveness, thereby its mutation might contribute to tumor growth and progress (17). According to a larger number of previous studies, EGFR TKI plays a critical role in the antitumor effects among NSCLC patients with EGFR mutation by binding to tyrosine kinase to control EGFR enzymatic activity and repress signal conduction, subsequently decreasing the growth of tumor cell (18). As a novel target EGFR TKI, icotinib is the first Chinese small molecular targeted antineoplastic drug, which has similar chemistry structure and working mechanism with gefitinib and erlotinib (19). However, compared to these two popular EGFR-TKI, icotinib may have more advantages as follows: (I) it has better capability to penetrate cytomembrane and blood brain barrier (BBB) by lipid solubility (19); (II) it has better selectivity and competitiveness to bind to kinases, thereby effectively blocking signal conduction (5); (III) it could be metabolized by various enzymes, including CYP2C19, CYP3A4, and CYP2E1, thereby reducing drug accumulation and decreasing toxicity (13,20).
Accumulating studies have investigated the efficacy of EGFR-TKI in NSCLC patients, suggesting that EGFR-TKI is optimal choice as the first-line therapy in advanced NSCLC patients, particular in patients with EGFR-mutation (6,9). A clinical trial, OPTIMAL compares the efficacy of erlotinib and chemotherapy and illustrates that CR of 2% and ORR of 83% in erlotinib group are higher than CR of 0% and ORR of 36% in control group (21). Based on ICOGEN trial, a phase III trial, which compared icotinib with gefitinib in second-line therapy, indicates that icotinib achieved ORR of 27.6%, which is similar to gefitinib group with ORR of 27.2% (13). Moreover, a previous study found that first-line icotinib treatment achieved CR of 0.0% and ORR of 62.9% among advanced NSCLC with EGFR-sensitive mutation, and there were only 35 patients enrolled in this previous study and the sample size was relatively small, thereby lacking statistical power (5). In the present study, we enrolled a larger sample size with 152 advanced NSCLC patients with EGFR-sensitive mutation and assessed the treatment response of first-line icotinib treatment, which revealed that the rates of CR and ORR were 4% and 71.8% respectively, which was higher compared to ICOGEN and OPTIMAL trials. The possible reasons were that: (I) all patients enrolled in our study were with EGFR-sensitive mutation, who might have better response to EGFR TKIs, including icotinib; (II) icotinib is applied as first-line treatment in advanced NSCLC patients with EGFR-sensitive mutation in this study.
In the current study, we also observed that median PFS and OS were 14.0 months (95% CI: 12.7–15.3 months) and 33.0 months (95% CI: 24.9–41.1 months) respectively. The survival duration of patients treated with icotinib in our study was relatively long compared to gefitinib and erlotinib in NSCLC patients with EGFR mutation in several clinical trials: in OPTIMAL trials, median PFS is 13.1 months (95% CI: 10.58–16.53 months); in LUX-lung 3 trials, median PFS is 13.6 months for afatinib and 6.0 months for chemotherapy (HR, 0.47; 95% CI, 0.34–0.65; P=0.001); in LUX-lung 6 trial, PFS is prolonged in patients received afatinib compared to patients received gemcitabine or patients received cisplatin (median PFS 11.0 vs. 5.6 months, HR =0.28; P<0.0001) (21,22). In addition, a respective study recruited 49 NSCLC patients with wild-type or with EGFR mutation, which showed that the PFS was 9.5 months (95% CI: 7.9–11.0 months) and 8.5 months (95% CI: 6.5–10.5 months) in patients treated with first-line and second-line/further-line icotinib respectively (15). Also, a randomized phase III study of docetaxel plus cisplatin versus pemetrexed plus cisplatin as first-line treatment in NSCLC patients discloses that the median PFS and OS are 4.7 months (95% CI: 4.4–5.0 months) and 11.7 months (95% CI, 8.6–14.8 months) in the Pem-Cis arm, and 4.4 months (95% CI, 3.7–5.1 months) and 13.3 months (95% CI, 8.1–18.5 months) in the Doc-Cis arm (23). Another interesting previous study suggested that the median PFS and OS were 11.0 months (95% CI: 10.2–11.8 months) and 21.0 months (95% CI: 20.1–21.9 months) respectively in NSCLC patients with EGFR mutation received first-line icotinib treatment (5). Therefore, these results suggest that first-line icotinib treatment is efficient in advanced NSCLC patients with EGFR-sensitive mutation. The possible reasons for better survival duration in this study were that: (I) all patients enrolled in this study were with EGFR-sensitive mutation, who might have better response to EGFR TKIs, including icotinib, and icotinib is applied as first-line treatment in these patients; (II) if PD occurred, some of them would receive repeated icotinib treatment if it was beneficial after evaluation, some of them would receive icotinib treatment with a higher dose, and others would receive further treatment such as chemotherapy, third-generation EGFR-TKIs and optimum supportive treatment, thereby prolonging survival duration in advanced NSCLC patients with EGFR-sensitive mutation.
In the current study, we also observed that smoking history was statistically correlated with non-ORR achievement in advanced NSCLC patients with EGFR mutation received first-line icotinib treatment. This might result from that smoking history is negatively associated with EGFR mutation, thereby improving efficacy of icotinib (24). Moreover, the results of this study showed that EGFR mutation 19del was statistically associated with ORR achievement in advanced NSCLC patients with EGFR mutation received first-line icotinib treatment, which had the similar trend with the results of a meta-analysis, which assesses clinical efficacy of icotinib in lung cancer patients with different EGFR mutation status and discloses that compared to EGFR L858R patients, EGFR 19Del patients treated with icotinib have better ORRs, and it is a useful biomarker to assess the efficacy of icotinib in advanced NSCLC patients (14). However, no factor affecting PFS and OS was observed in the current study, the possible reasons were as follows: (I) sample size was relatively small in this study, thus the influence of extremum may lead to the excursion of efficacy in subgroups; (II) the follow-up duration was short, and the median value was only 15.0 (range: 1.0–44.0) months; (III) all the patients enrolled in this study were in TNM stage IIIb and IV, While the prognosis of NCLC patients was greatly affected by TNM stage, thus the influence of other factors would be reduced relatively.
With respect to safety, most common AEs including rash, diarrhea and pain have been reported in NSCLC patients, and most AEs were with mild grade (5,13). In accordance of these studies, we also observed that icotinib with related AEs mainly involved rash, diarrhea and abnormal liver function and the most AEs were in mild grade. In addition, there was no severe drug toxicity, such as myelosuppression. Therefore, it suggested that icotinib was well tolerated in advanced NSCLC patients with EGFR-sensitive mutation.
There were still patients who needed further treatment (patients were SD or PD). To these patients, nearly half of them received the secondary tissue biopsy or liquid biopsy as evidence for further treatments, including repeated icotinib treatment, chemotherapy, third-generation EGFR-TKIs, optimum supportive treatment or other treatments, suggesting that if PD occurred, patients treated with first-line icotinib therapy still could receive further treatment.
Despite the interesting results reported, there were still some limitations existing in this study. Firstly, it was a retrospective study and all patients enrolled were from eastern China, thus, a cross-regional study with larger sample size is necessary. Secondly, follow-up duration was relatively short, the potential long-term efficacy of icotinib as first-line therapy in advanced NSCLC patients with EGFR-sensitive mutation is needed to be addressed in further study.
In conclusion, first-line icotinib treatment was efficient and well tolerated in patients with advanced NSCLC harboring EGFR mutations, and smoking history was negatively while EGFR mutation 19del was positively correlated with ORR achievement.
Acknowledgments
Funding: None.
Footnote
Conflict of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.05.30). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by Ethics Committee of Shanghai Chest Hospital, Shanghai Jiao Tong University. The number of ethical approval was KS1034. The written informed consents or oral agreements (with recording) were required from all patients or their guardians.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Torre LA, Siegel RL, Jemal A. Lung Cancer Statistics. Adv Exp Med Biol 2016;893:1-19. [Crossref] [PubMed]
- Reck M, Heigener DF, Mok T, et al. Management of non-small-cell lung cancer: recent developments. Lancet 2013;382:709-19. [Crossref] [PubMed]
- Shen YW, Zhang XM, Li ST, et al. Efficacy and safety of icotinib as first-line therapy in patients with advanced non-small-cell lung cancer. Onco Targets Ther 2016;9:929-35. [Crossref] [PubMed]
- Yoon SM, Shaikh T, Hallman M. Therapeutic management options for stage III non-small cell lung cancer. World J Clin Oncol 2017;8:1-20. [Crossref] [PubMed]
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92-8. [Crossref] [PubMed]
- Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213-22. [Crossref] [PubMed]
- Fiala O, Pesek M, Finek J, et al. Comparison of EGFR-TKI and chemotherapy in the first-line treatment of advanced EGFR mutation-positive NSCLC. Neoplasma 2013;60:425-31. [Crossref] [PubMed]
- Krawczyk P, Kowalski DM, Ramlau R, et al. Comparison of the effectiveness of erlotinib, gefitinib, and afatinib for treatment of non-small cell lung cancer in patients with common and rare EGFR gene mutations. Oncol Lett 2017;13:4433-44. [Crossref] [PubMed]
- Murray S, Dahabreh IJ, Linardou H, et al. Somatic mutations of the tyrosine kinase domain of epidermal growth factor receptor and tyrosine kinase inhibitor response to TKIs in non-small cell lung cancer: an analytical database. J Thorac Oncol 2008;3:832-9. [Crossref] [PubMed]
- Tan F, Shi Y, Wang Y, et al. Icotinib, a selective EGF receptor tyrosine kinase inhibitor, for the treatment of non-small-cell lung cancer. Future Oncol 2015;11:385-97. [Crossref] [PubMed]
- Shi Y, Zhang L, Liu X, et al. Icotinib versus gefitinib in previously treated advanced non-small-cell lung cancer (ICOGEN): a randomised, double-blind phase 3 non-inferiority trial. Lancet Oncol 2013;14:953-61. [Crossref] [PubMed]
- Qu J, Wang YN, Xu P, et al. Clinical efficacy of icotinib in lung cancer patients with different EGFR mutation status: a meta-analysis. Oncotarget 2017;8:33961-71. [Crossref] [PubMed]
- Song Z, Yu X, Cai J, et al. Efficacy of icotinib for advanced non-small cell lung cancer patients with EGFR status identified. Zhongguo Fei Ai Za Zhi 2013;16:138-43. [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Kumar A, Petri ET, Halmos B, et al. Structure and clinical relevance of the epidermal growth factor receptor in human cancer. J Clin Oncol 2008;26:1742-51. [Crossref] [PubMed]
- Tan F, Shen X, Wang D, et al. Icotinib (BPI-2009H), a novel EGFR tyrosine kinase inhibitor, displays potent efficacy in preclinical studies. Lung Cancer 2012;76:177-82. [Crossref] [PubMed]
- Ma XH, Tian TD, Liu HM, et al. Efficacy and influence factors of icotinib hydrochloride in treating advanced non-small cell lung cancer. Eur Rev Med Pharmacol Sci 2017;21:266-74. [PubMed]
- Liu D, Jiang J, Zhang L, et al. Metabolite characterization of a novel anti-cancer agent, icotinib, in humans through liquid chromatography/quadrupole time-of-flight tandem mass spectrometry. Rapid Commun Mass Spectrom 2011;25:2131-40. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [Crossref] [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [Crossref] [PubMed]
- Park CK, Oh IJ, Kim KS, et al. Randomized Phase III Study of Docetaxel Plus Cisplatin Versus Pemetrexed Plus Cisplatin as First-line Treatment of Nonsquamous Non-Small-cell Lung Cancer: A TRAIL Trial. Clin Lung Cancer 2017;18:e289-96. [Crossref] [PubMed]
- Dogan S, Shen R, Ang DC, et al. Molecular epidemiology of EGFR and KRAS mutations in 3,026 lung adenocarcinomas: higher susceptibility of women to smoking-related KRAS-mutant cancers. Clin Cancer Res 2012;18:6169-77. [Crossref] [PubMed]


