Prognostic significance of CD47 in human malignancies: a systematic review and meta-analysis
Introduction
CD47, also known as integrin-associated protein (IAP), is a transmembrane protein belonging to the immunoglobulin superfamily (1). CD47 is widely expressed on the surfaces of normal cells, especially hematopoietic cells. It interacts with signal regulatory protein alpha (SIRPα) to activate a signaling cascade in various phagocytic cells, including dendritic cells and macrophages (2). CD47 is overexpressed in a variety of hematopoietic malignancies and solid tumors, and appears to promote tumor proliferation, progression, and metastasis (3-5).
Numerous studies have associated high CD47 expression with poor survival, suggesting CD47 could be used as a prognostic factor in various cancers, including leukemia (6), prostate cancer (7), lung cancer (8) and hepatocellular carcinoma (9). However, conflicting results have also been reported (10,11), making the prognostic utility of CD47 in malignancies uncertain (12-14). We performed a meta-analysis to assess the value of CD47 overexpression for predicting clinical outcomes, and to examine the association between CD47 and clinicopathological parameters in solid tumors.
Methods
Literature search
We systematically searched PubMed, Embase, Web of Science, and the Cochrane library for studies published before July 28, 2017 that evaluated the effects of CD47 expression in various cancers. The MeSH terms and text words included “CD47”, “CD47 Antigens”, “Integrin-Associated Protein p50”, “Thrombospondin-1 Receptor CD47”, “neoplasm”, “malignancy”, “cancer”, “carcinoma”, “tumor”, “prognostic”, “outcomes”, and “survival”. We also manually screened the references from included articles to identify additional potentially relevant studies.
Inclusion and exclusion criteria
Eligible studies satisfied the following inclusion criteria: (I) Iassessed CD47 expression in predicting prognosis in any type of human cancer; (II) used a cohort design; (III) CD47 was associated with OS, DFS, EFS, and PFS, and HRs with 95% CIs were directly reported or could be calculated from the data; and (IV) patients were divided into high and low CD47 expression groups.
Studies were excluded based on the following criteria: (I) conference abstracts, reviews, letters, editorials, or case reports; (II) experiments not performed on patient tissues; and (III) did not provide data for calculating HRs and 95% CIs. If multiple articles included data from the same patients, we included only the most informative and recent study.
Date extraction and quality assessment
Two investigators (HJ Zhao, F Pan) independently extracted data from included studies and assessed study qualities. Discrepancies were solved through a third investigator (YC Shi). The following data were recorded: first author’s name, publication year, country, cancer type, sample size, follow-up time, tissues, detection method, cut-off value, analysis type, HR calculation method, outcome measures with HRs and corresponding 95% CIs, and clinicopathological parameters in solid tumors (such as patient sex, age, tumor differentiation, lymph node metastasis, distant metastasis, and TNM stage). For studies providing clinical outcome data, multivariate HRs were calculated prior to univariate HRs to assess the prognostic value of CD47 expression. For articles containing only Kaplan-Meier curves, survival data was extracted using Engauge Digitizer v.4.1, and HRs and 95% CIs were estimated using Tierney’s method (15).
Article qualities were evaluated using the Newcastle-Ottawa scale (NOS). Scores ranged from 0–9, and we defined studies with NOS scores ≥7 as high quality. Studies included in this meta-analysis scored between 5 and 8.
Statistical analysis
HRs with corresponding 95% CIs were utilized to assess relationships between CD47 levels and patient clinical outcomes using Stata v.14.0 software (Stata Corporation, College Station, TX, USA). Odds ratios (ORs) were calculated to analyze associations between CD47 expression and clincopathological parameters using RevMan v.5.3 software. Statistical heterogeneity between studies was evaluated using Cochran’s Q test and Higgins I-squared statistic (I2). P<0.05 (Q test) and/or I2>50% indicated heterogeneity. A random effects or fixed effects model was applied to pool HRs in the absence or presence of heterogeneity; a pooled HR >1 with P<0.05 indicated poor prognosis in patients with increased CD47 expression. Subgroup analyses were conducted to evaluate the effects of individual factors on the association between CD47 and patient prognosis. Sensitivity analysis was performed to evaluate the robustness of the meta-analysis results. Funnel plots and Begg’s (rank correlation) and Egger’s (regression asymmetry) tests were used to estimate publication bias (16). If publication bias did exist, the trim and fill method was performed to assess its influence on overall outcomes (17).
Results
Study characteristics
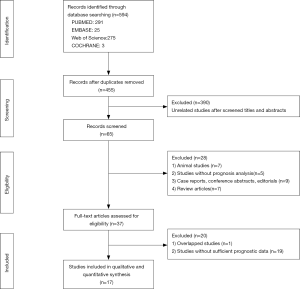
A total of 594 studies describing CD47 in various cancers were retrieved using our search strategy through different combinations of key terms (Figure 1). A total of 139 articles were excluded as duplicates, and 390 were excluded after screening titles and abstracts for unrelated studies. Of the remaining 65 studies, 17 articles containing 38 cohort studies (6,7,10,11,18-30) were included in this meta-analysis (Table 1). The 38 cohorts included 7,229 total patients, and study sample sizes ranged from 20–1,117 patients. The studies were performed in five countries, including China, Japan, Germany, Switzerland, and the United States, and were published between 2009 and 2014. The studies included 12 different cancer types, with two acute lymphoblastic leukemia (ALL) (18), six acute myeloid leukemia (AML) (6), six non-Hodgkin lymphoma (NHL) (28), three lung cancer (LC) (19), one melanoma (20), seven ovarian cancer (21-24), three glioma (7), one glioblastoma (7), five breast cancer (25-27), one esophageal squamous cell carcinoma (ESCC) (29), two gastric cancer (GC) (10,11), and one osteosarcoma cohort (30). Seventeen (44.7%) cohorts were conducted in Asian populations, and 21 (55.3%) in non-Asians. The association between CD47 expression and survival was analyzed as overall survival (OS) in 32 cohorts (6,7,10,11,18-24,26-30), disease-free survival (DFS) in three cohorts (18,27,29), event-free survival (EFS) in five cohorts (6,28), and progression-free survival (PFS) in eight cohorts (7,19,20,28,30). CD47 detection methods differed between studies, and included immunohistochemical (IHC) staining, real time polymerase chain reaction (real time PCR), and Affymetrix arrays or other cDNA microarrays. CD47 cut-off values were inconsistent across different studies. Hazard ratios (HRs) for OS were directly reported for 22 cohorts and were calculated indirectly using the Kaplan-Meier curves provided for 10 cohorts.
Full table
Association between CD47 expression and OS
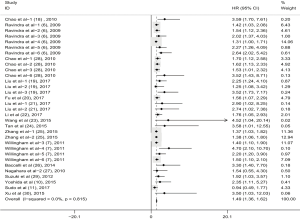
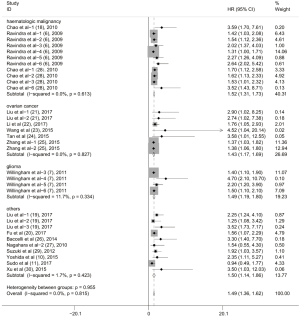
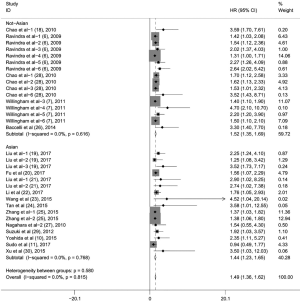
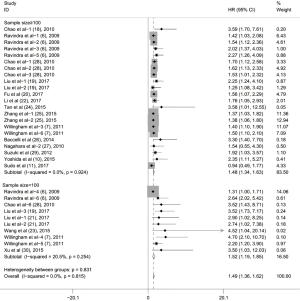
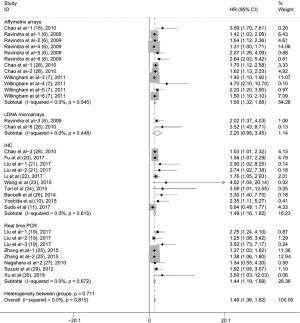
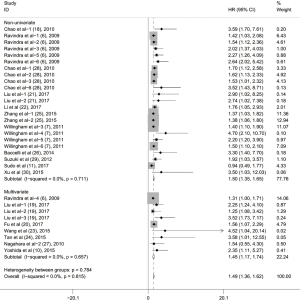
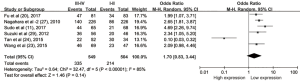
To evaluate the relationship between CD47 expression and OS in cancer patients, we assessed 6261 patients in 32 cohorts. The fixed effect model was applied to calculate pooled HRs with corresponding 95% confidence intervals (CIs), because we found no significant heterogeneity among studies (I2=0.00%, P=0.815). High CD47 expression was associated with unfavorable OS (HR =1.49; 95% CI: 1.36–1.62, P<0.001) (Figure 2).
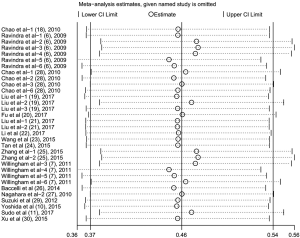
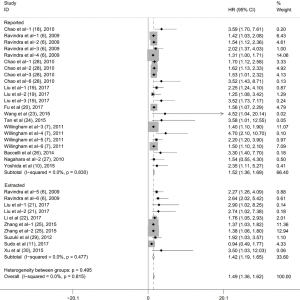
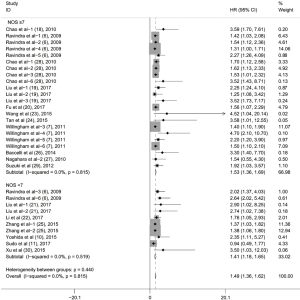
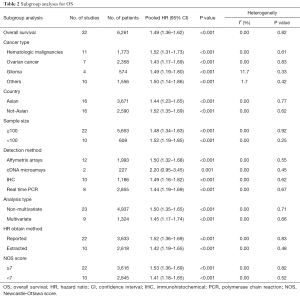
Subgroup analyses according to cancer type indicated that high CD47 expression was associated with worse OS in hematologic malignancies (HR =1.52; 95% CI: 1.31–1.73, P<0.001), ovarian cancer (HR =1.43; 95% CI: 1.17–1.69, P<0.001), and glioma (HR =1.49; 95% CI: 1.19–1.80, P<0.001), and other cancers (HR =1.50; 95% CI: 1.14–1.86, P<0.001) (Table 2, Figure S1). Subgroup analyses were also performed based on country, sample size, detection method, analysis type, HR obtained method and NOS score (Tables 2,S1, Figures S2-S7). The pooled cDNA microarray results were less persuasive, possibly because only two cohorts were included in the analysis. The remaining combined HR values from the above subgroups were >1.00 (P<0.001). We found no significant heterogeneity. To further assess the stability of the pooled outcomes, we performed a sensitivity analysis for OS. Removal of any single study had no significant effect on the pooled HRs (Figure 3).
Full table
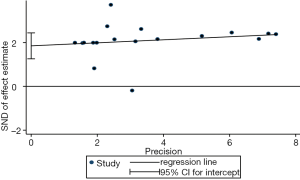
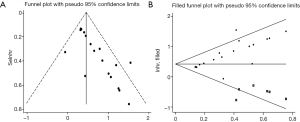
We evaluated publication bias for all included studies using a vertical funnel plot and Begg’s and Egger’s tests. The funnel plot appeared asymmetrical (Figure 4A), implying potential publication bias, and Begg’s test (P<0.001) and Egger’s tests (P<0.001) (Figure S8) revealed publication bias. The trim and fill method was then used to assess the influence of this bias on the overall outcome. A symmetrical funnel plot was generated using the estimated hypothetical negative studies (Figure 4B). The adjusted pooled HRs, including 11 hypothetical studies, showed an association between elevated CD47 and poor OS (HR =1.53; 95% CI: 1.33–1.75, P<0.001). Trim and fill indicated that pooled HRs for OS were robust.
Association between CD47 expression and DFS, EFS, and PFS
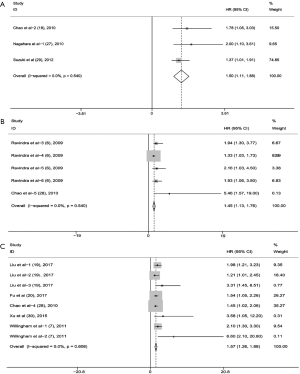
In this meta-analysis, three cohorts (18,28,29) including 750 patients reported an association between high CD47 expression and DFS. No heterogeneity was observed between studies (I2=0.00%, P=0.540). Thus, we applied a fixed model to estimate the pooled HRs with corresponding 95% CIs. Pooled HRs revealed a potential positive association between high CD47 expression and poor DFS (HR =1.50; 95% CI: 1.11–1.89, P<0.001) (Figure 5A).
Two articles (6,28) involving five cohorts and 514 patients suggested an association between CD47 expression and EFS. The combined HRs showed that increased CD47 expression might be associated with worse EFS (HR =1.45; 95% CI: 1.13–1.76, P<0.001) (Figure 5B).
Five articles (7,19,20,28,30) involving eight cohorts and 889 patients reported an association between CD47 expression and PFS. The pooled HRs showed that elevated CD47 expression was associated with worse PFS (HR =1.57, 95% CI: 1.26–1.88, P<0.001) (Figure 5C).
Associations between CD47 expression and clinicopathological parameters
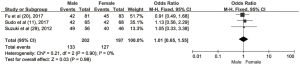
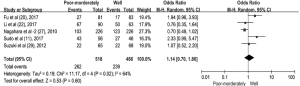
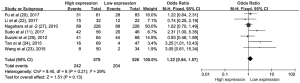
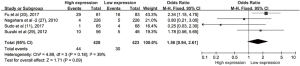
The pooled ORs and 95% CIs for clinicopathological parameters in solid tumors are shown in Table 3. There was no significant correlation between CD47 and gender, tumor differentiation, lymph node metastasis, distant metastasis, or TNM stage (P>0.05) (Figures S9-S13). The influence of CD47 expression on other clinicopathological parameters in solid tumors could not be determined, as insufficient data was provided.
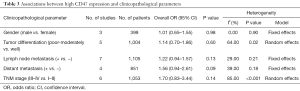
Full table
Discussion
CD47 is a transmembrane protein belonging to the immunoglobulin superfamily and was first identified as a biomarker for human ovarian cancer in the 1980s (31,32). It is also widely expressed on the surfaces of normal cells, and is overexpressed in many types of cancer (1,33). SIRPα belongs to a multi-gene family of immune receptors, and is expressed on myeloid and neuronal cells in the central nervous system (34,35). CD47 could allow cancer cells to escape immune surveillance by activating a signaling cascade through SIRPα in phagocytic cells (33). The CD47-SIRPα axis is a critical regulator of myeloid cell activation and functions as a myeloid-specific immune checkpoint (6). High CD47 expression is associated with advanced prognosis in many cancer types (6,7,10,18-30), and blocking the CD47-SIRPα interaction promotes cancer cell eradication by phagocytes (36).
We performed the present meta-analysis to assess the relationship between CD47 expression and tumor patient prognosis. Our analysis included 17 articles with 38 cohorts and 7,229 cases. We found that increased CD47 expression was associated with poor OS. Subgroup analyses revealed that CD47 overexpression was associated with poor OS stratified by cancer type, country, sample size, detection method, analysis type, HR obtained method and NOS score, without obvious heterogeneity. However, pooled results for cDNA microarrays were less persuasive, because only two cohorts used this method. Our findings indicated that cancer patients with elevated CD47 have worse OS, DFS, EFS, and PFS, and that CD47 could be a prognostic biomarker for patients with different cancers. CD47 might thus be an efficacious anti-cancer therapeutic target.
Previous studies showed that blocking the CD47-SIRPα interaction contributes to cancer cell eradication by stimulating macrophage phagocytosis and anti-tumor immune responses (6,12,28). Phagocytosis could induce secretion of cytokines and chemokines, recruiting additional immune cells to tumors, and amplifying the therapeutic effects of CD47 blockade (36). Myeloid immune cells (13), such as monocytes, granulocytes (37), and dendritic cells (38), and non-myeloid immune cells, such as T cells and natural killer (NK) cells, may also respond to CD47/SIRPα-blocking therapies (39). CD47 blockade appears to initiate or reinforce adaptive immune responses in murine models of lymphoma, lung cancer, and ovalbumin (OVA)-expressing MC38 tumors (14,37). CD47 also induces caspase-independent cell death by regulating intercellular signaling, indicating that direct cytotoxicity to CD47+ tumor cells might be a mechanism of action in CD47-targeting therapies (40,41).
To our knowledge, ours is the first meta-analysis associating high CD47 expression with poor prognosis in patients with various cancers. However, our analysis had several limitations. First, data were limited for some types of solid tumors, such as lung cancer, breast cancer, gastric cancer, glioblastoma, and melanoma, which reduced the statistical power of our findings. Second, detection method and cut-off value were inconsistent across the included studies, which may have resulted in statistical heterogeneity. Third, HRs extracted from Kaplan-Meier curves might not be as reliable as those reported directly in articles, and might introduce errors. Additionally, publication bias with respect to OS may have resulted from diverse data formats and the preference for positive results across publications. The funnel plot and Begg’s and Egger’s tests suggested the existence of publication bias, in part because few studies report negative results. However, trim and fill showed that the corrected pooled HRs did not reverse the pooled results, indicating that the positive correlation between high CD47 level and poor prognosis was not completely caused by a lack of negative results in the included studies. Still, more objective studies reporting both positive and negative results are needed to improve our analysis. Finally, our study size was relatively small and additional data are needed to strengthen our reporting power.
Conclusions
Our results indicated that CD47 could serve as a useful biomarker to predict prognosis in cancer patients, and is a potential therapeutic target. Further data from large-scale, well-designed studies are needed to confirm our results.
Full table
Acknowledgments
Funding: This work is supported by Natural Science Foundation of China (Grant number 2015AA020701), National High-tech Research and Development Projects (863), to Key Technology for gut microbiota.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.05.31). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Oldenborg PA, Gresham HD, Lindberg FP. CD47-signal regulatory protein alpha (SIRPalpha) regulates Fcgamma and complement receptor-mediated phagocytosis. J Exp Med 2001;193:855-62. [Crossref] [PubMed]
- Brown EJ, Frazier WA. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol 2001;11:130-5. [Crossref] [PubMed]
- Chan KS, Espinosa I, Chao M, et al. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc Natl Acad Sci U S A 2009;106:14016-21. [Crossref] [PubMed]
- Edris B, Weiskopf K, Volkmer AK, et al. Antibody therapy targeting the CD47 protein is effective in a model of aggressive metastatic leiomyosarcoma. Proc Natl Acad Sci U S A 2012;109:6656-61. [Crossref] [PubMed]
- Sick E, Boukhari A, Deramaudt T, et al. Activation of CD47 receptors causes proliferation of human astrocytoma but not normal astrocytes via an Akt-dependent pathway. Glia 2011;59:308-19. [Crossref] [PubMed]
- Majeti R, Chao MP, Alizadeh AA, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 2009;138:286-99. [Crossref] [PubMed]
- Willingham SB, Volkmer JP, Gentles AJ, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A 2012;109:6662-7. [Crossref] [PubMed]
- Zhao H, Wang J, Kong X, et al. CD47 Promotes Tumor Invasion and Metastasis in Non-small Cell Lung Cancer. Sci Rep 2016;6:29719. [Crossref] [PubMed]
- Xiao Z, Chung H, Banan B, et al. Antibody mediated therapy targeting CD47 inhibits tumor progression of hepatocellular carcinoma. Cancer Lett 2015;360:302-9. [Crossref] [PubMed]
- Yoshida K, Tsujimoto H, Matsumura K, et al. CD47 is an adverse prognostic factor and a therapeutic target in gastric cancer. Cancer Med 2015;4:1322-33. [Crossref] [PubMed]
- Sudo T, Takahashi Y, Sawada G, et al. Significance of CD47 expression in gastric cancer. Oncol Lett 2017;14:801-9. [Crossref] [PubMed]
- Matlung HL, Szilagyi K, Barclay NA, et al. The CD47-SIRPalpha signaling axis as an innate immune checkpoint in cancer. Immunol Rev 2017;276:145-64. [Crossref] [PubMed]
- Adams S, van der Laan LJ, Vernon-Wilson E, et al. Signal-regulatory protein is selectively expressed by myeloid and neuronal cells. J Immunol 1998;161:1853-9. [PubMed]
- Liu X, Pu Y, Cron K, et al. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat Med 2015;21:1209-15. [Crossref] [PubMed]
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [Crossref] [PubMed]
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [Crossref] [PubMed]
- Duval S, Tweedie R. Trim and Fill:A simple Funnel-Plot-Based method of testing and adjusting for publication bias in meta-Analysis. Biometrics 2000;56:455-63. [Crossref] [PubMed]
- Chao MP, Alizadeh AA, Tang C, et al. Therapeutic Antibody Targeting of CD47 Eliminates Human Acute Lymphoblastic Leukemia. Cancer Res 2011;71:1374-84. [Crossref] [PubMed]
- Liu L, Zhang L, Yang L, et al. Anti-CD47 antibody as a targeted therapeutic agent for human lung cancer and cancer stem cells. Front Immunol 2017;8:404. [Crossref] [PubMed]
- Fu W, Li J, Zhang W, et al. High expression of CD47 predicts adverse prognosis in Chinese patients and suppresses immune response in melanoma. Biomed Pharmacother 2017;93:1190-6. [Crossref] [PubMed]
- Liu R, Wei HT, Gao P, et al. CD47 promotes ovarian cancer progression by inhibiting macrophage phagocytosis. Oncotarget 2017;8:39021-32. [PubMed]
- Li Y, Lu SH, Xu Y, et al. Overexpression of CD47 predicts poor prognosis and promotes cancer cell invasion in high-grade serous ovarian carcinoma. Am J Transl Res 2017;9:2901-10. [PubMed]
- Wang H, Tan MZ, Zhang S, et al. Expression and Significance of CD44, CD47 and c-met in Ovarian Clear Cell Carcinoma. Int J Mol Sci 2015;16:3391-404. [Crossref] [PubMed]
- Tan M, Zhu LC, Zhuang HY, et al. Lewis Y antigen modified CD47 is an independent risk factor for poor prognosis and promotes early ovarian cancer metastasis. Am J Cancer Res 2015;5:2777-87. [PubMed]
- Zhang H, Lu HQ, Xiang LS, et al. HIF-1 regulates CD47 expression in breast cancer cells to promote evasion of phagocytosis and maintenance of cancer stem cells. Proc Natl Acad Sci U S A 2015;112:E6215-23. [Crossref] [PubMed]
- Baccelli I, Stenzinger A, Vogel V, et al. Co-expression of MET and CD47 is a novel prognosticator for survival of luminal-type breast cancer patients. Oncotarget 2014;5:8147-60. [Crossref] [PubMed]
- Nagahara M, Mimori K, Kataoka A, et al. Correlated expression of CD47 and SIRPA in bone marrow and in peripheral blood predicts recurrence in breast cancer patients. Clin Cancer Res 2010;16:4625-35. [Crossref] [PubMed]
- Chao MP, Alizadeh AA, Tang C, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell 2010;142:699-713. [Crossref] [PubMed]
- Suzuki S, Yokobori T, Tanaka N, et al. CD47 expression regulated by the miR-133a tumor suppressor is a novel prognostic marker in esophageal squamous cell carcinoma. Oncol Rep 2012;28:465-72. [Crossref] [PubMed]
- Xu JF, Pan XH, Zhang SJ, et al. CD47 blockade inhibits tumor progression human osteosarcoma in xenograft models. Oncotarget 2015;6:23662-70. [Crossref] [PubMed]
- Poels LG, Peters D, van Megen Y, et al. Monoclonal antibody against human ovarian tumor-associated antigens. J Natl Cancer Inst 1986;76:781-91. [PubMed]
- Epenetos AA, Munro AJ, Stewart S, et al. Antibody-guided irradiation of advanced ovarian cancer with intraperitoneally administered radiolabeled monoclonal antibodies. J Clin Oncol 1987;5:1890-9. [Crossref] [PubMed]
- Weiskopf K. Cancer immunotherapy targeting the CD47/SIRPalpha axis. Eur J Cancer 2017;76:100-9. [Crossref] [PubMed]
- van Beek EM, Cochrane F, Barclay AN, et al. Signal regulatory proteins in the immune system. J Immunol 2005;175:7781-7. [Crossref] [PubMed]
- Seiffert M, Cant C, Chen Z, et al. Human signal-regulatory protein is expressed on normal, but not on subsets of leukemic myeloid cells and mediates cellular adhesion involving its counterreceptor CD47. Blood 1999;94:3633-43. [PubMed]
- Weiskopf K, Ring AM, Ho CC, et al. Engineered SIRPalpha variants as immunotherapeutic adjuvants to anticancer antibodies. Science 2013;341:88-91. [Crossref] [PubMed]
- Zhao XW, van Beek EM, Schornagel KV. CD47-signal regulatory protein-α (SIRPα) interactions form a barrier for antibody-mediated tumor cell destruction. Proc Natl Acad Sci U S A 2011;108:18342-7. [Crossref] [PubMed]
- Yi T, Li J, Chen H, et al. Splenic Dendritic Cells Survey Red Blood Cells for Missing Self-CD47 to Trigger Adaptive Immune Responses. Immunity 2015;43:764-75. [Crossref] [PubMed]
- Soto-Pantoja DR, Terabe M, Ghosh A, et al. CD47 in the tumor microenvironment limits cooperation between anti-tumor T cell immunity and radiation therapy. Cancer Res 2014;74:6771-83. [Crossref] [PubMed]
- Mateo V, Brown EJ, Biron G, et al. Mechanisms of CD47-induced caspase-independent cell death in normal and leukemic cells:link between phosphatidylserine exposure and cytoskeleton organization. Blood 2002;100:2882-90. [Crossref] [PubMed]
- Kikuchi Y, Uno S, Kinoshita Y, et al. Apoptosis inducing bivalent single-chain antibody fragments against CD47 showed antitumor potency for multiple myeloma. Leuk Res 2005;29:445-50. [Crossref] [PubMed]