
Clinicopathologic features and prognostic factors of diffuse sclerosing variant of papillary thyroid carcinoma: a population-based analysis
Introduction
Differentiated thyroid cancer is the most common endocrine malignancy and most are papillary thyroid carcinomas (PTCs). Although the prognosis of PTC is always excellent with a 10-year survival rate over 90%, some patients suffer tumor recurrence and even death (1). In the past few decades, several histologic variants including diffuse sclerosis variant (DSV) and tall cell variant (TCV) were identified to be responsible for the aggressive behavior compared to classical PTC. DSVPTC was first described in 1985 by Vickery et al., and it was recognized as a new histological variant of PTC in 1988 by the World Health Organization (WHO) (2,3). The prevalence of DSVPTC is low at approximately 0.4–6% (4). DSVPTC has aggressive pathological features such as diffuse involvement of one or both thyroid lobes, extrathyroidal extension (ETE) and lymph node metastasis (LNM) (5-7). Nevertheless, due to the low prevalence of DSVPTC and the fact that previous studies were case reports and single-center case series, it is debatable whether DSVPTC has a poorer prognosis compared with classical PTC, and even the rate of distant metastasis differs from 5% to 60% (8). Thus, the appropriate approach for treating DSV is still not certain.
The objectives of this study were to perform a population-based analysis of the biological aggressiveness of DSV. To our knowledge, this is the first such population-based report. It included 331 cases of DSVPTC and 63,659 cases of classical PTC. We compared clinicopathological features and outcomes of DSVPTC patients with classical PTC patients, and further analyzed risk factors associated with prognosis.
Methods
Data sources and study subjects
For this study, data were extracted from the Surveillance, Epidemiology, and End Results (SEER) 18 database of the National Cancer Institute, which collects information on cancer incidence and survival from 18 population-based cancer registries, including 28% of the USA population.
The National Cancer Institute’s SEER*Stat software (Version 8.3.4; Surveillance Research Program, National Cancer Institute, Bethesda, MD, USA; www.seer.cancer.gov/seerstat) was used to identify patients with classical PTC and DSV from 2004 to 2013 using ICD-O-3 codes 8050, 8260, and 8341 (classical PTC); 8350 (DSVPTC); and 8050, 8052, 8130, 8260, 8340–8344, 8350, 8450 and 8460 (all PTC). In total, 332 DSVPTCs and 64,387 classical PTCs were diagnosed during 2004 and 2013. The exclusion criteria were as follows: (I) the presence of secondary malignancies, and (II) any deficiency of clinicopathologic data including gender, age, race, tumor size, ETE, LNM, distant metastasis (DM), surgical procedure, therapeutic regimens and survival. Ultimately, 331 DSVPTCs and 63,659 classical PTCs were included.
Primary data extracted from the database for analysis included gender, age at diagnosis, race, tumor size, multifocality, ETE, cervical LNM, DM, surgical procedure, adjuvant therapies, cause of death and survival in months. Age at diagnosis was divided into three groups: ≤18, 19–54 and ≥55 years. Race was classified into white, black and other (American Indian, Alaska Native, Asian, Pacific Islander, and other unspecified). All of the enrolled patients with PTC were staged pathologically according to the 8th edition of the American Joint Committee on Cancer (AJCC) pTNM system. Tumor size was defined as the diameter of the largest focus in the thyroid and was classified into four groups: 0.1–1, 1.1–2, 2.1–4 and >4 cm. Clinically, multifocal lesions were defined as two or more cancer sites within the thyroid (9). Tumor extension was treated as intrathyroidal and ETE. Multifocal lesions, ETE and LNM were all assessed in the final pathology. The pathologic status of LNM was divided into negative (lymph nodes examined were all negative) and positive. According to the AJCC of differentiated thyroid cancer, the stage of LNM was further divided into pN1a [central LNM (CLNM)] and pN1b [lateral LNM (LLNM)].
Patients who underwent subtotal, near-total, or completion thyroidectomy after having had a prior partial resection were considered to have undergone a total thyroidectomy (TT). Patients who underwent local tumor destruction, removal of less than a lobe, lobectomy, removal of a lobe or partial removal of the contralateral lobe were considered to have undergone other surgery procedures. Primary outcomes were defined as overall survival (OS: month from diagnosis to death from any cause) and cancer-specific survival (CSS: months from diagnosis to death due to cancer).
Statistical analysis
The chi-square test and analysis of variance were used to analyze categorical and continuous variables respectively. Fischer’s exact test was used to analyze categorical variables with expected values less than 5. Cox regression was used to distinguish the independent predictors of CSS. Survival was analyzed using the Kaplan-Meier method, and the log-rank test was used to determine if differences in survival were statistically significant. Variables with P<0.1 on univariate analysis were included in multivariate analysis. All P values were two-sided. P<0.05 was considered statistically significant. Statistical analysis was performed using SPSS software, version 21.0 (SPSS Inc., Chicago, IL, USA).
Results
There were 331 cases of DSV and 63,659 cases of classical PTC diagnosed during 2004 and 2013. In this study, DSVPTCs accounted for 0.35% (331/93,611) of all PTC patients. The mean age of DSVPTC patients in this study was 44.3±18.1 years (range, 6.0–86.0 years) and the classical PTC patients was 48.8±15.4 years (range, 3.0–98.0 years). Patients with DSVPTC and classical PTC were followed for up to 10 years. Mean follow-up for DSVPTC and classical PTC was 6.0 and 6.3 years, respectively.
Clinicopathologic characteristics
Clinical and pathologic characteristics are summarized in Tables 1,2. DSVPTCs afflicted a higher proportion of women (81.6% vs. 76.6%, P=0.033) and young patients than classical PTCs (≤18 years: 9.4% vs. 1.5%, P<0.001). There were no significant demographic differences between patients with DSVPTC and classical PTC with respect to race. Patients with DSVPTC had TT and lymph node dissection (LND) more frequently compared to classical PTC (TT: 91.2% vs. 82.9%, P<0.001; LND: 70.7% vs. 53.0%, P<0.001). Patients with DSVPTC were more likely to receive radioiodine therapy (RAT) (60.4% vs. 47.9%, P<0.001). However, there were no differences between DSVPTC and classical PTC with respect to rates of DM (2.4% vs. 1.2%, P=0.055). Among the eight DSVPTCs with DM, lung metastasis occurred in seven patients and bone metastasis in one patient.
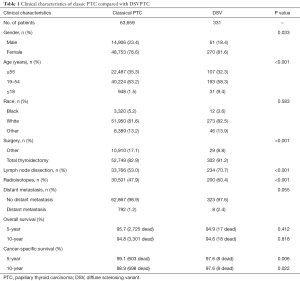
Full table
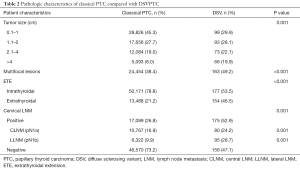
Full table
Regarding the pathological features of DSVPTCs, the tumor size was larger on average compared to classical PTCs (20.66 vs. 16.54 mm, P<0.001), with significantly higher rates of ETE (46.5% vs. 21.2%, P<0.001), multifocal lesions (38.4% vs. 49.2% P<0.001). Moreover, 52.9% of DSVPTC patients had positive lymph nodes pathologically, of which 24.2% were pN1a and 28.7% were pN1b. The rate of cervical LNM was significantly higher than that of classical PTCs (26.8%, P=0.001).
Survival
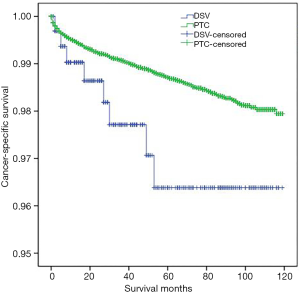
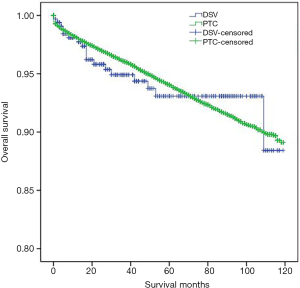
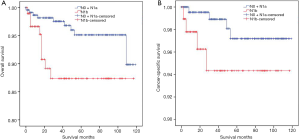
DSVPTC had a worse CSS than classical PTC according to the univariate log-rank test (P=0.025) (Figure 1). The 5-year CSS was 97.6% in the DSV group and 99.1% in the classical PTC group (P=0.006), and the 10-year CSS was 97.6% in the DSVPTC group and 98.9% in the classical PTC group (P=0.022) (Table 1). Univariate and multivariate cox regression analysis of CSS in patients with DSV and classical PTC showed that DSV, the pathologic subtype of PTC itself, was the only independent predictor for CSS (P<0.05) (Table 3). The difference in OS between DSVPTC and classical PTC was not statistically significant (P=0.904) (Figure 2).
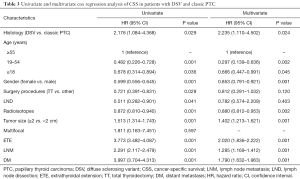
Full table
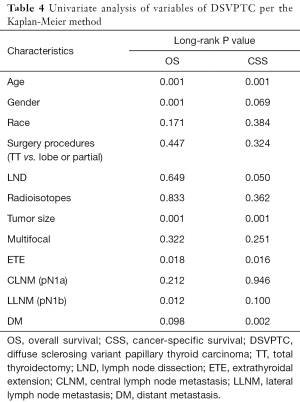
Predictors of OS and CSS in patients with DSV
Univariate analysis identified ≥55 years of age, male gender, tumor size, ETE, pN1b and DM as significant factors for both OS and CSS while radioisotope was another significant factor for CSS (Table 4). When multivariate analysis with Cox regression was performed, the variables that were validated as independent prognostic factors for OS included: ≥55 years of age (HR 7.024, 95% CI: 2.311–21.352) and pN1b (HR 3.088, 95% CI: 1.051–9.070). The variables that were validated as independent prognostic factors for CSS included: ≥55 years of age (HR 19.432, 95% CI: 6.353–57.418), ≥2 cm tumor size (HR 10.193, 95% CI: 1.160–89.595), ETE (HR 24.521, 95% CI: 1.378–436.325), pN1b (HR 11.590, 95% CI: 1.019–131.844) and DM (HR 34.920, 95% CI: 1.493–816.669) (Table 5).
Full table

Full table
LLNM in DSV
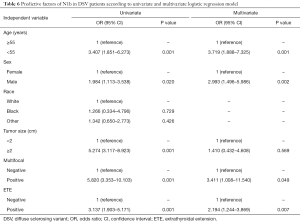
LLNM (pN1b) occurred much more frequently in DSVPTCs than classical PTCs (28.7% vs. 9.9%, P=0.001), and then univariate and multivariate logistic regression were performed to find the risk factors for pN1b in DSVPTC patients (Table 6). Univariate analysis demonstrated that ≥55 years of age, male, ≥2 cm tumor size, multifocality and ETE were correlated with pN1b, whereas multivariate analysis identified only ≥55 years of age, male, ≥2 cm tumor size and ETE as independent risk factors for pN1b.
Full table
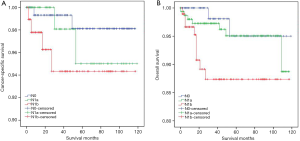
Since LLNM (pN1b) was an independent risk factor for survival in DSVPTC patients, further analyses of survival curve were conducted among DSVPTC patients who were divided into groups according to the cervical lymph node status (pN0, pN1a and pN1b). Although there was no significant difference of CSS (P=0.211, Figure 3A) among the three groups [5-year CSS, N1b 95.79% (91/95), N1a 97.50% (78/80), N0 98.72% (154/156)], DSV patients with pN1b had a worse OS than the other two groups [5-year OS: N1b 90.53% (86/95), N1a 97.50% (78/80), N0 96.15% (150/156); P=0.039, Figure 3B]. On the other hand, OS and CSS were poorer in the pN1b group than both the pN0 and pN1a groups (P=0.012, P=0.041, respectively; Figure 4A,B).
Discussion
The prevalence of DSVPTC ranges from 0.4–6.6% among patients with PTC (4,6). In this study, DSVPTCs accounted for 0.35% (331/93,611) of all PTC patients. DSVPTC is often recognized to have a young patient age, and it was even reported as the most common subtype of pediatric PTC (5). Compared with classical PTCs in this study, DSVPTC patients were younger and included a higher proportion of patients under 18 (9.4%, P=0.001). This study also showed a high female-to-male ratio in DSVPTC, which was similar to previous reports.
Our results demonstrate that DSVPTCs showed aggressive clinicopathological characteristics with larger tumor size and increased rates of multifocality, ETE and lymph node involvement. Previous studies reported the same manifestations (10,11). The American Thyroid Association (ATA) even classified DSVPTC as an intermediate- or high-risk group (7). In a multicentric study, Chereau et al. even found that these unfavorable features were more frequent in DSV than in high-risk variants of PTCs (8). Histopathologically, papillary structures in dilated lympho-vascular spaces are often present. The tumors show extensive squamous metaplasia, abundant psammoma bodies, stromal fibrosis and prominent lymphocytic infiltration (12). In immunohistochemical studies, DSVPTC shows different expression patterns of epithelial membrane antigen, galectin 3, cell adhesion molecules, p53 and p63 compared to conventional PTC. On genetic analysis, the occurrence of BRAF and RAS mutations are uncommon events in DSVPTCs but RET/PTC rearrangement is the major genetic alteration (4,13). Furthermore, Joung et al. (14) reported that DSVPTC with RET/PTC3 presented more frequently with T4 or M1 stage disease, and tumors with RET/PTC3 were more likely to occur in younger patients. DSVPTC with RET/PTC1 has been associated with a higher remission rate (14). Considering such clinical manifestations, TT is recommended as the standard operation for DSVPTCs.
Regarding lymph node involvement, the rate of LNM in DSVPTCs is up to 80.3–96%, and 86% of DSVPTCs were reportedly pN1b postoperatively (8,10). In our study, LNM (pN1) was observed in 52.9% of DSVPTCs postoperatively but in 26.8% of classical PTCs, and LND was carried out more frequently in patients with DSVPTC than classical PTCs (70.7% vs. 53.0%, P=0.001). The most common site of DM in DSV patients is the lung, which is similar to our results, and the incidence of DM is 7% to 14.9% (8,10). However, a low rate of DM was observed in our study, and there was no significant difference between DSV and classical PTC (2.4% vs. 1.2%, P=0.055). The low rate of DM of DSV found in our study could be explained by the higher rate of TT and LND followed by RAT performed in DSV patients. Thus, we are in favor of performing TT and prophylactic central LND (CLND) combined with RAT postoperatively, and a careful and long-term follow-up is necessary.
Although DSVPTCs are often associated with more advanced AJCC stage and higher risk of recurrence compared with classical PTCs, the prognosis of DSVPTC is not certain. Several researchers found a high survival rate of DSVPTC, and there was no significant difference in cancer-specific mortality between DSVPTCs and classical PTCs (6,15-18). This might be because DSVPTC tends to occur among younger patients who respond well to the treatment (10). On the other hand, Akaishi et al. found that the 10-year disease-free survival (DFS) rate of DSVPTC was significantly lower than that of classical PTC (60.5% vs. 88.6%) (6). In a large-scale meta-analysis conducted by Vuong et al., DSV patients displayed a compromised OS, in line with Qahtani et al. (10,19). In our study, there was no difference in OS between DSVPTC and classical PTC, but DSVPTC had a poorer CSS (5-year CSS: 97.6% vs. 99.1%, P=0.006; 10-year CSS: 97.6% vs. 98.9%, P=0.022). To the best of our knowledge, since the current study included the largest number of DSV patients with systematic analysis, we demonstrated that the histological type of DSV itself was the only independent predictor for CSS (P<0.05, Table 3).
Lateral LND (LLND) is not recommended for classical PTCs according to the ATA guidelines (5). However, in our study, DSVPTCs presented a high rate of LNM, especially in the lateral compartment. The rate of LLNM (pN1b) in DSVPTC patients was nearly 3 times that of classical PTC patients (28.7% vs. 9.9%, P=0.001). Multivariate analysis demonstrated that LLNM (pN1b) was an independent prognostic factor for both OS and CSS of DSVPTCs, and the results of survival analyses among DSVPTCs showed that pN1b group had a worse prognosis than the pN1a and pN0 groups. All this evidence indicates that LLNM (pN1b) plays a significant role in predicting the prognosis of DSVPTCs among all those prognostic factors. Therefore, we think that surgeons should give full consideration to LND in DSVPTC patients with such predictors as ≥55 years of age, male, ≥2 cm tumor size and ETE. It requires further study to evaluate prophylactic LND for DSVPTCS with risk factors of LLNM.
Although this is the largest study so far, it still has several limitations, mainly its retrospective nature. The SEER database has some intrinsic deficiencies including incomplete data for some variables, lack of data on variables not collected by SEER (such as features of imaging examinations, molecular pathogenesis (BRAF/RET gene status), the extent of lymphadenectomy, recurrence, and response of RAT to distant metastatic foci). Since DSVs are rare and newly identified tumors, we should document more detailed differences with other variants of PTC to improve our understanding of DSV.
Conclusions
This study demonstrated that DSVs are commonly seen in young patients and are a group of high-risk PTCs with aggressive clinicopathologic characteristics including larger tumor size, multifocality, ETE and lymph node involvement which might predict a high rate of recurrence. DSVPTC patients with risk factors such as ≥55 years, ≥2 cm tumor size, ETE, LLNM (pN1b) and DM might develop a poor prognosis. TT and prophylactic central neck dissection followed by RAT are more likely to be performed in patients with DSV while careful surveillance should be emphasized postoperatively. Since LLNM presented frequently and was identified as a risk factor for survival in patients with DSVPTC, surgeons should give full consideration to LND in DSVPTC patients with the following risk factors: ≥55 of age years, male, ≥2 cm of tumor size, and ETE. Overall, outcomes of DSV are similar to classical PTC, but this variant displays worse CSS. Long-term data and higher-powered studies are required to demonstrate the prognosis of DSV patients.
Acknowledgments
Funding: This study was funded by the Shanghai Municipal Planning Commission of Science and Research Fund for Young Scholar (award number 20154Y0050).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.05.47). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional ethical approval and informed consent were waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 2006;295:2164-67. [Crossref] [PubMed]
- Vickery AL Jr, Carcangiu ML, Johannessen JV, et al. Papillary carcinoma. Semin Diagn Pathol 1985;2:90-100. [PubMed]
- Hedinger C, Williams ED, Sobin LH. The WHO histological classification of thyroid tumors: a commentary on the second edition. Cancer 1989;63:908-11.
- Pillai S, Gopalan V, Smith RA, et al. Diffuse sclerosing variant of papillary thyroid carcinoma–an update of its clinicopathological features and molecular biology. Crit Rev Oncol Hematol 2015;94:64-73. [Crossref] [PubMed]
- Fukushima M, Ito Y, Hirokawa M, et al. Clinicopathologic characteristics and prognosis of diffuse sclerosing variant of papillary thyroid carcinoma in Japan: an 18-year experience at a single institution. World J Surg 2009;33:958-62. [Crossref] [PubMed]
- Akaishi J, Sugino K, Kameyama K, et al. Clinicopathologic features and outcomes in patients with diffuse sclerosing variant of papillary thyroid carcinoma. World J Surg 2015;39:1728-35. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Chereau N, Giudicelli X, Pattou F, et al. Diffuse sclerosing variant of papillary thyroid carcinoma is associated with aggressive histopathological features and a poor outcome: results of a large multicentric study. J Clin Endocrinol Metab 2016;101:4603-10. [Crossref] [PubMed]
- Lu Z, Sheng JD, Zhang YJ, et al. Clonality analysis of multifocal papillary thyroid carcinoma by using genetic profiles. J Pathol 2016;239:72-83. [Crossref] [PubMed]
- Vuong HG, Kondo T, Pham TQ, et al. Prognostic significance of diffuse sclerosing variant papillary thyroid carcinoma: a systematic review and meta-analysis. Eur J Endocrinol 2017;176:431-39. [Crossref] [PubMed]
- Kuo EJ, Goffredo P, Sosa JA, et al. Aggressive variants of papillary thyroid microcarcinoma are associated with extrathyroidal spread and lymph-node metastases: A population-level analysis. Thyroid 2013;23:1305-11. [Crossref] [PubMed]
- Lloyd RV, Buehler D, Khanafshar E. Papillary thyroid carcinoma variants. Head Neck Pathol 2011;5:51-6. [Crossref] [PubMed]
- Sheu SY, Schwertheim S, Worm K, et al. Diffuse sclerosing variant of papillary thyroid carcinoma: lack of BRAF mutation but occurrence of RET/PTC rearrangements. Mod Pathol 2007;20:779-87. [Crossref] [PubMed]
- Joung JY, Kim TH, Jeong DJ, et al. Diffuse sclerosing variant of papillary thyroid carcinoma: major genetic alterations and prognostic implications. Histopathology 2016;69:45-53. [Crossref] [PubMed]
- Regalbuto C, Malandrino P, Tumminia A, et al. A diffuse sclerosing variant of papillary thyroid carcinoma: clinical and pathologic features and outcomes of 34 consecutive cases. Thyroid 2011;21:383-89. [Crossref] [PubMed]
- Albareda M, Puig-Domingo M, Wengrowicz S, et al. Clinical forms of presentation and evolution of diffuse sclerosing variant of papillary carcinoma and insular variant of follicular carcinoma of the thyroid. Thyroid 1998;8:385-91. [Crossref] [PubMed]
- Kazaure HS, Roman SA, Sosa JA. Aggressive variants of papillary thyroid cancer: incidence, characteristics and predictors of survival among 43,738 patients. Ann Surg Oncol 2012;19:1874-80. [Crossref] [PubMed]
- Malandrino P, Russo M, Regalbuto C, et al. Outcome of the diffuse sclerosing variant of papillary thyroid cancer: a meta-analysis. Thyroid 2016;26:1285-92. [Crossref] [PubMed]
- Alqahtani K, Asiri M, Tunio M, et al. Diffuse Sclerosing Variant Papillary Thyroid Carcinoma: Clinicopathological and Treatment Outcome Analysis of 44 Cases. Kuwait Med J 2015;47:225-30.


