
The eligibility of primary tumor resection for de novo stage IV breast cancer patients
There are many retrospective reports to indicate the survival benefit of primary tumor resection for de novo stage IV breast cancer. Moreover, several comprehensive reviews have described significant differences in survival time (hazard ratio of ~0.6) (1,2). However, retrospective reports include numerous biases. Patients undergoing surgery might be in good condition for surgery, while those not receiving surgical treatment might have poor overall condition. In addition, details regarding the efficacy and the disease control rate for systemic therapy are lacking. Currently, we don’t know the most appropriate timing for surgery or patients’ status. One of the most important questions is whether the best timing for primary tumor resection is before or after primary systemic therapy. We can determine the indications of surgery according to the efficacy of systemic therapy. Moreover, we can completely remove the locally advanced primary tumor with muscle and/or skin invasion after tumor volume reduction by systemic therapy. Lane et al. reported the results of patterns of surgical care and their association with overall survival among a contemporary cohort of women with stage IV breast cancer (3). They reported that surgical resection of the primary tumor occurs in almost half of women with stage IV breast cancer alive 1 year after diagnosis. Primary tumor resection for de novo stage IV breast cancer patients, especially after systemic therapy (before systemic therapy: hazard ratio, 0.62–0.73; after systemic therapy: hazard ratio, 0.56; P<0.001), was independently associated with improved adjusted overall survival when compared to systemic therapy alone. However, there were no details about the response to primary systemic therapy. The Translational Breast Cancer Research Consortium (TBCRC) reported results of a prospective cohort study of stage IV breast cancer patients (4). There was no significant prognostic effect of primary tumor resection for responders to primary systemic therapy. We need to confirm the indications and timing of surgery by prospective randomized trials.
Five prospective randomized trials have analyzed the efficacy of primary tumor resection for stage IV breast cancer (Table 1) (5). Three have reported final results, and the others are still currently enrolling patients or following up. The first results were from the Indian trial (6). The efficacy of primary tumor resection for Stage IV breast cancer patients with sensitivity to primary systemic therapy was evaluated, and they could not indicate the prognostic efficacy of surgery. A Turkish trial (MF07-01) (7) and the ABCSG-28 (POSYTIVE trial) (8) evaluated prognostic effects of surgery as the primary treatment before systemic therapy. The Turkish trial suggested a positive effect of primary surgery; however, the POSYTIVE trial could not demonstrate an OS benefit. We cannot get the best evidence from the prospective studies so far because these trials have had limitations in the evaluations; In the Indian trial, systemic therapies were not selected according to breast cancer subtypes. Anti-HER2 molecular targeted therapies were not used for patients with HER2-positive tumors, and very few patients with ER-positive tumors received hormone administration as primary systemic therapy. The Regatta trial for stage IV gastric cancer reported similar results. Gastrectomy followed by chemotherapy yielded no survival benefit compared with chemotherapy alone (9). The authors suggested one of the reasons was reduced compliance with chemotherapy after surgery due to adverse events like weight loss. We think the most important treatment of metastatic cancer is effective drug from these trials’ results.
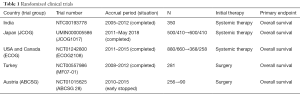
Full table
Moreover, discontinuation of effective systemic chemotherapy after randomization might result in a poorer outcome in distant progression-free survival in the patients with surgery group. This result follows the pattern of previous reports. Folkman demonstrated that the primary tumor actively secretes angiostatin, which suppresses the angiogenic activity of metastatic cancer, and that resection of the primary tumor removes that suppression, and thus increases angiogenesis and growth of metastatic lesions (10). Fisher demonstrated that animals with metastatic disease were immunologically compromised, and that surgical stress releases growth factors, which in turn stimulate proliferation of metastasized cancer cells (11). The POSYTIVE trial reported similar results; however, there were no details about systemic therapy after randomization. More data on systemic treatment are needed to evaluate this clinical question. Given the POSYTIVE trial lacking the statistical power, and the Turkish trial has not published some details, a discussion about the discordance of results between these trials is warranted.
The most important treatment for stage IV breast cancer patients is systemic therapy. The improvement of systemic drugs prolongs survival absolutely. Local therapy, including surgery, is one of the choices for metastatic breast cancer treatment. Currently, there are no definitive results to evaluate the prognostic effect of surgery. The Turkish trial indicated a positive effect, but the Indian trial reported a worse effect of surgery for these patients. From the results of these prospective studies, primary tumor resection for de novo stage IV breast cancer cannot be recommended to all patients routinely. The impact of surgery on survival is not so large for de novo stage IV breast cancer. We need to consider eligibility for surgery and planning integrated treatment strategies, including local therapy, according to breast cancer subtype, metastases and the patient’s condition. Our aim should be to devise the most effective treatment strategies for individual cancer patients, employing drugs, surgery and radiation, alone or in combination. The treatment goals for stage IV breast cancer are to prolong the patient’s survival time and to control symptoms.
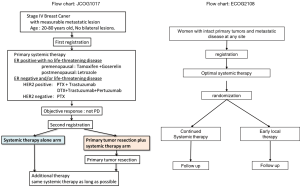
The Japan Clinical Oncology Group (JCOG 1017) and Eastern Clinical Oncology Group (ECOG 2108) are enrolling and following patients for a phase 3 trial (Figure 1). In these trials, patients received the most up-to-date standard systemic therapy available before and after randomization, and also the most advanced form of imaging examination available before treatment. It is anticipated that the aforementioned trials will resolve current controversies and provide many eagerly awaited answers.
Acknowledgments
Funding: This report was supported in part by the National Cancer Center Research and Development Fund (29-A-3) from the Ministry of Health, Labour and Welfare and the Practical Research for Innovative Cancer Control (18ck0106307h0002) from Japan Agency for Medical Research and Development, AMED.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Xiao-Wei Qi (Third Military Medical University, Chongqing, China).
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.05.28). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Petrelli F, Barni S. Surgery of primary tumors in stage IV breast cancer: an updated meta-analysis of published studies with meta-regression. Med Oncol 2012;29:3282-90. [Crossref] [PubMed]
- Ruiterkamp J, Voogd AC, Bosscha K, et al. Impact of breast surgery on survival in patients with distant metastases at initial presentation: a systematic review of the literature. Breast Cancer Res Treat 2010;120:9-16. [Crossref] [PubMed]
- Lane WO, Thomas SM, Blitzblau RC, et al. Surgical Resection of the Primary Tumor in Women With De Novo Stage IV Breast Cancer: Contemporary Practice Patterns and Survival Analysis. Ann Surg 2017; [Epub ahead of print]. [Crossref] [PubMed]
- King TA, Lyman JP, Gonen M, et al. Prognostic impact of 21-gene recurrence score in patients with Stage IV breast cancer: TBCRC 013. J Clin Oncol 2016;34:2359-65. [Crossref] [PubMed]
- Shien T, Nakamura K, Shibata T, et al. A randomized controlled trial comparing primary tumour resection plus systemic therapy with systemic therapy alone in metastatic breast cancer (PRIM-BC): Japan Clinical Oncology Group Study JCOG1017. Jpn J Clin Oncol 2012;42:970-3. [Crossref] [PubMed]
- Badwe R, Hawaldar R, Nair N, et al. Locoregional treatment versus no treatment of the primary tumour in metastatic breast cancer: an openlabel randomised controlled trial. Lancet Oncol 2015;16:1380-8. [Crossref] [PubMed]
- Soran A, Ozmen V, Ozbas S, et al. A randomized controlled trial evaluating resection of the primary breast tumor in women presenting with de novo stage IV breast cancer: Turkish Study (Protocol MF07-01). J Clin Oncol 2016;34:abstr 1005.
- Fitzal F, Bjelic-Radisic V, Knauer M, et al. Impact of Breast Surgery in Primary Metastasized Breast Cancer: Outcomes of the Prospective Randomized Phase III ABCSG-28 POSYTIVE Trial. Ann Surg 2018. [Epub ahead of print].
- Fujitani K, Yang HK, Mizusawa J, et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single noncurable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol 2016;17:309-18. [Crossref] [PubMed]
- Folkman J. New perspectives in clinical oncology from angiogenesis research. Eur J Cancer 1996;32A:2534-9. [Crossref] [PubMed]
- Fisher ER, Fisher B. Experimental studies of factors influencing the development of hepatic metastases. XIII. Effect of hepatic trauma in parabiotic pairs. Cancer Res 1963;23:896-900. [PubMed]


