Clinical implication of platelet-lymphocyte ratio and PD-L1 in breast cancer patients
Introduction
Tumor microenvironment has been considered to be associated with tumor development and aggressiveness (1,2). Immune cells compromising the tumor microenvironment mediate immune response in the existence of tumor and influence the prognosis of cancer patients (3). The programmed death 1/programmed death ligand-1 (PD-1/PD-L1) pathway which regulates T-cell activation and differentiation and helps tumor cells to escape host immune system (4) has been widely reported as an unfavorable prognostic factor in breast cancer in these recent years (5-7). Besides, studies support that PD-L1 expression correlates to low tumor infiltrating lymphocytes (TILs) (8)and PD-L1 expression combined with low tumor-infiltrating CD8+ lymphocytes density is associated with aggressive clinical outcome (9). Consistently, in our previous study, we have demonstrated that intratumor PD-L1 expressed in 21.7% of all breast cancer, and most of them were triple negative breast cancer (TNBC). Most importantly, patients with PD-L1 expression achieved worse disease-free survival (DFS) and overall survival (OS) than those without PD-L1 expression in breast cancer (10). A reported study (11) found serum soluble PD-L1 and biomarkers of the host immunity which included neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR) and systemic immune-inflammation index (SII) could predict clinical outcome of biliary tract cancer (BTC) patients receiving palliative chemotherapy. Combinations of different biomarkers that can reveal tumor-immune reaction and host immunity are likely to be more appropriate to evaluate the patients’ outcome.
NLR and PLR have been considered to reflect the general immune status of the host and proposed as prognostic markers in various cancers (12-17). High NLR is widely considered as a poor prognostic factor in breast cancer (18). Some studies consider PLR is not associated with clinical outcome of breast cancer patients (19,20), while a meta-analysis including seven studies with 3,741 patients conclude high PLR is associated with shorter DFS and OS in breast cancer patients (21). Combination of PD-L1 and other parameters has been reported in breast cancer (9,22,23). However, combination of NLR, PLR, and PD-L1 in predicting prognosis in breast cancer has not been reported. So in this study, we will evaluate the prognostic role of the combination of pre-treatment NLR, PLR, and PD-L1 in breast cancer.
Methods
Patients
A total of 870 breast cancer patients treated in Sun Yat-sen University Cancer Center from 2000 to 2012 with suitable paraffin-embedded tumor samples for PD-L1 test were included. All patients fulfilled the following criteria: (I) breast cancer with pathological confirmation in our center; (II) underwent surgery according to routine clinical practice in our center; (III) underwent adjuvant chemotherapy and/or endocrine therapy according to routine clinical practice in our center; (IV) with known PD-L1 status. We retrospectively collected clinicopathological data including pre-treatment complete blood count (CBC), age, menstruation status, tumor size, histological grade, lymph nodes, estrogen receptor (ER) status, progesterone receptor (PR) status, human epidermal receptor 2 (HER2) status, pathological staging according to American Joint Committee on Cancer (AJCC) 7th edition, DFS, cancer-specific survival (CSS), and OS.
DFS was defined as the time from curative surgery to occurrence of any disease progression resulting in inoperable, locoregional recurrence, distant metastasis, or death of any reason; CSS was defined as the time from curative surgery to death of breast cancer; OS was defined as the time from curative surgery to death of any reason. PLR was defined as absolute platelet count in the peripheral blood before treatment divided by absolute lymphocyte count; NLR was defined as the absolute neutrophil count in the peripheral blood before treatment divided by absolute lymphocyte count; all results reserved 2 digits after the decimal point.
PD-L1 immunohistochemical staining
Slides were stained using a Ventana Discovery XT automated system (Ventana Medical Systems, Tucson, AZ, USA) with proprietary reagents according to the manufacturer’s protocol as we had mentioned in the published paper (10). Procedures were elaborated in supplementary material. Staining of over 5% of tumor cell membrane with or without cytoplasm staining was considered as positive.
Statistics
X-Tile was used to access the optimal cut-off value of NLR and PLR. Patients were randomly separated into training set and validation set to verify the cut-off value. Then patients were classified into high PLR/NLR and low PLR/NLR groups according to the cut-off value. Baseline characteristics between two groups were compared using chi-square test and Mann-Whitney U test. The OS and PFS were assessed using Kaplan-Meier estimates. The log-rank test was used to compare differences in DFS and OS among groups. The Cox proportional hazards model with 95% confidence interval (CI) was used for the Univariate analysis to verify the factors significantly related to DFS and OS. Multivariate Cox model was constructed to adjust for other clinical characteristics that were significant in the univariate analyses. Results were considered statistically significant if P value of 0.05 or less. All analyses were performed using X-Tile (3.6.1; Rimm lab, Yale School of Medicine, New Haven) and SPSS software for Windows (version 13; IBM SPSS, Somers, NY, USA).
Results
Patients’ characteristics
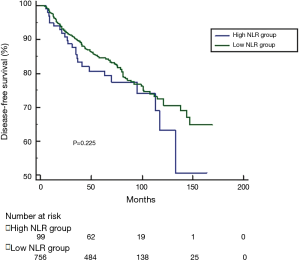
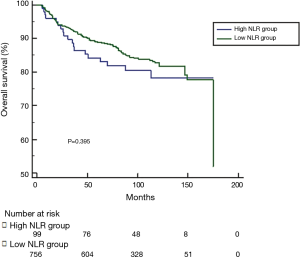
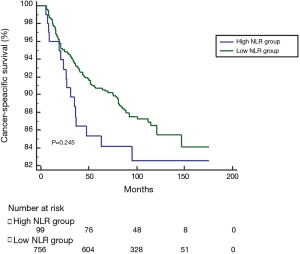
The optimal cut-off value for NLR generated from training set was 3.00, however, this cut-off value didn’t show significance in the validation set (P=0.343). According to 3.00, we classified patients into high NLR group and low NLR group. As expected, no significance was shown in DFS, CSS or OS between groups (see Figures S1-S3). For this reason, no further analysis of NLR was done. The optimal cut-off value of PLR was 147.50 and showed significance in the validation set (P=0.045) (Figure S4). A total of 212 patients with pretreatment PLR >147.50 were classified into high PLR group, while 658 patients with PLR ≤147.50 were in low PLR group. Baseline characteristics were compared between groups.
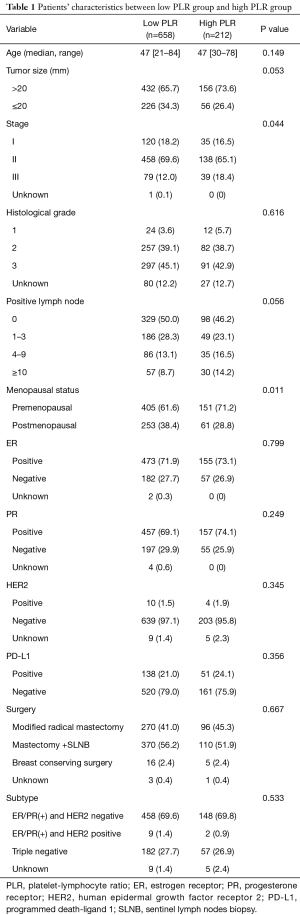
Intratumor PD-L1 expressed in 192 (21.7%) patients, in which 129 (67.2% of all PD-L1 positive patients) were TNBC as we reported in the previous study (10). The baseline characteristics between two groups were shown in Table 1. The median age of 870 patients with breast cancer was 47 years old in both groups (P=0.149). Most patients (69.7%) are ER/PR positive. Higher percentage of stage III patients in high PLR group (18.4%) than that in low PLR group (12.0%) (P=0.044). Patients with high PLR were more likely to be associated with premenopausal status (P=0.011). There were no significant differences in tumor size, histological grade, positive lymph node number, ER, PR, HER2, PD-L1 expression, and molecular subtype between both groups.
Full table
Survival of the patients
The median follow-up was 96 months (range from 6 to 265 months). High PLR group showed shorter DFS compared to the low PLR group (Figure 1A). The 5-year DFS rate was 78.7% in high PLR group and 85.6% in low PLR group (P=0.003). Likewise, patients in high PLR group significantly achieved worse result than those in low PLR group in median time to CSS and OS (Figure 1B,C). The 5-year survival rate was 82.6% in high PLR group and 88.8% in low PLR group (P=0.010).
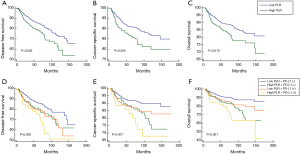
In order to see whether patients with PD-L1 expression and high pre-treatment PLR would have a poorer prognosis, we classified all patients into high PLR + PD-L1(+) group, high PLR + PD-L1(−) group, low PLR + PD-L1(+) group and low PLR + PD-L1(−) group. We found that patients with PD-L1 expression and high pretreatment PLR had the worst prognosis (Figure 1D,E,F). The 5-year DFS rates were 68.4%, 78.8%, 76.8% and 85.8% respectively (P=0.002). The 5-year OS rates were 73.4%, 82.6%, 85.2% and 90.1% respectively (P<0.001).
Prognostic factor for DFS and OS
PLR, PD-L1 expression and patients’ characteristics including age, menopausal status, tumor size, lymph node status, ER status, PR status, HER2 status, NLR were analyzed in the univariate and multivariate analysis for DFS and OS. High pretreatment PLR was an independent prognostic factor for both DFS (adjusted HR =1.525, 95% CI: 1.111–2.094, P=0.009) (Table 2) and OS (adjusted HR =1.527, 95% CI: 1.073–2.174, P=0.019) (Table 3). PD-L1 expression was associated with poor DFS and OS. In addition, both tumor size and lymph node status were independent predictors for DFS and OS.
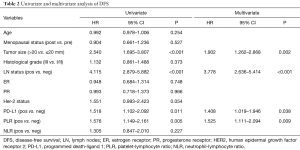
Full table
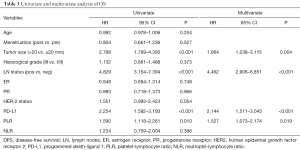
Full table
In order to see whether the combination of PD-L1 and PLR would better predict survivals of the patients, we conducted hazard ratio model including clinicopathologic characteristics. Combination of high PLR and PD-L1(+) showed higher adjusted HR for both DFS (adjusted HR: 2.294, 95% CI: 1.372–3.831) and OS (adjusted HR: 3.345, 95% CI: 1.919–5.848, P<0.001). Interestingly, we found that combination of high PLR and PD-L1(−) was not associated with DFS (HR: 1.575, 95% CI: 0.895–2.770, P=0.635) in the univariate analysis but an independent factor for OS (adjusted HR: 2.247, 95% CI: 1.219–4.132, P=0.009) (Table 4). Tumor size and lymph node status were still independent factors for both DFS and OS.

Full table
Discussion
Our study demonstrated that patients in high pre-treatment PLR were associated with shorter DFS and OS compared to those in low PLR group. We also addressed that PLR and PD-L1 were independent prognostic factors for DFS and OS after adjusting for tumor size, lymph node status and PD-L1 status.
Several studies have shown high PLR is associated with poor prognosis in various cancers (24,25). A meta-analysis demonstrated high PLR was associated with an adverse outcome in breast cancer patients, which was consistent with our results. In most original studies, PLR is more often analyzed with NLR. However, unlike NLR, the role of PLR in predicting prognosis remained uncertain. Several mentioned meta-analysis indicated it was also a predictive factor in cancer patients’ clinical outcome. In this study, consisted of above results, we also demonstrated that PLR was an unfavorable prognostic factor for DFS and OS after adjusting for other factors. The most optimal cutoff value of PLR is still controversial. Previous studies used quintiles to classify patients into different groups and found 5-year survival rate in the highest group was the lowest compared to other quintiles (19). However, most methods for determination of cut-off value was manual and could not achieve randomized study. In our study, the cut-off value was generated from training and validated in validation dataset by X-tile, which may lessen manual confounding factors in some extents.
NLR failed to show association with patient survival in this study, which may due to the different cut-off value used (12). A presentation (26) in 2016 ESMO shows patients with early breast cancer in the GEICAM study with elevated derived neutrophil to lymphocyte ratio (dNLR) is associated with shorter DFS and OS in non-luminal subtype and shorter DFS in HER-2 enriched subtype defined by PAM50. And in this study, median and quartiles of NLR were used to classify patients into different groups.
The mechanism of PLR influencing prognosis in cancer is uncertain. Tumor-promoting inflammation is an important hallmark in cancer (27). Prior studies have reported platelets play some roles in tumor growth and tumor metastasis, like enhancing genomic instability, promoting tumor angiogenesis, protecting circulating tumor cell (CTC) from NK cell elimination, and promoting CTC adhesion on endothelium (28). Besides, lymphocytes have been implicated in playing important roles in tumor surveillance and anti-tumor activity (29). Leucocytes, as well as platelets, could be activated by tumor cells, and then cytokines, chemokines, and growth factors could be generated. Some of them promote tumor development and metastasis, while some of them inhibit tumor growth (30). It’s biologically likely that PLR may reflect the ability of the host immune reaction to the existing tumor, and imbalance of the ration may provide some insights of tumor progression and prognosis in some extent.
The PD-1/PD-L1 pathway is considered related to tumor cells escape from immune surveillance, tumor survival and progression (4). In our study, we found patients with high PLR and PD-L1 expression achieved the worst prognosis compared to other groups, on the contrary, the prognosis of patients with low PLR and PD-L1 negative was best. Patients with high PLR and PD-L1 negative had higher hazard ratio for OS than those only with high PLR. This information indicates the effect of combination PLR and PD-L1 is not simply added but may relate to complex underlying mechanisms. On one hand, high expression of PD-1/PD-L1 is common in many tumor cells, particularly in combination with inactivation of tumor suppressor genes (4). Tumor cells survive from evading immune surveillance through the PD-1/PD-L1 pathway. On the other hand, high counts of immune cells and platelets further promote tumor growth and aggressiveness. Besides, immune cells and platelets produce and secrete pro-inflammatory cytokines, such as interleukin-6, tumor necrotic factor-α, transforming growth factor-β, which can up-regulate expression of PD-L1 (31-33). In this way, these two factors may work together more efficiently in enhancing tumor aggressiveness.
Our previous study (10) showed PD-L1 higher expression was associated poorer clinicopathological characteristics, like larger tumor size, higher histological grade, more lymph node involved and ER/PR negative, which is consistent with reported studies (34,35). However, the prognostic role of PD-L1 is controversial. Studies of combinations of PD-L1 with other parameters in predicting prognosis are commonly reported as combination of PD-L1 and TILs. PD-L1 may be related to lower percentage of CD8+ TIL, which was likely to be associated with poor prognosis (22,36,37). On the contrary, some studies also have shown PD-1/PD-L1 expression on tumor TILs may be associated with favorable outcome in TNBC (38) and HER2 positive breast cancer (39). These results may be owing to the fact that PD-1/PD-L1 expression will be up-regulated physically when the immune reaction is too intensive, and also most studies only tested expression of PD-1/PD-L1 at some point in time. The previously mentioned study combines serum soluble PD-L1 with pre-treatment NLR and PLR in predicting clinical outcome of BTC patients (11). In this study, soluble PD-L1 and NLR were independent prognostic factors for OS, but the correlation of the two significant parameters and the role of the combination of these two parameters in prognosis were not analyzed. In our study, we showed PLR was an independent prognostic factor in breast cancer, and the worst prognosis was achieved in high PLR and PD-L1 expression group, which suggests subdivision of patients may provide more information and some of them may need different treatments. Considering the immunity function of platelets, combination of PLR and PD-L1 may provide some insights of novel studies or treatments. For example, a study (40) showed a novel approach to conjugate the PD-L1 antibody to platelets in order to improve the response rate of immunotherapy in post-surgical cancer patients. The activated platelets can deliver PD-L1 antibody to the surgical bed as well as CTC, and then PD-L1 antibody blocks PD-L1 and T cells can function normally to kill residual tumor cells. So in this situation, both activated platelet counts and PD-L1 expression in tumor beds must be crucial.
With the profound understanding of the interaction of immune system and cancer cells, it is essential and meaningful to investigate more valid parameters that may influence the prognosis of cancer patients. To our knowledge, this is the first study to evaluate the prognostic value of the combination of PLR and PD-L1 in breast cancer. Inevitably, there were some limitations in our study. first of all, this was a retrospective study so there may be an element of selection bias. Though we included patients meeting including criteria as much as we could, the final population showed relatively low proportion of Her-2 positive patients and a higher proportion of luminal subtype. This also led to Cox regression showed ER, PR, and Her-2 were not prognostic factors. Sample size will be expanded in the future study to investigate the prognostic value of PLR in Her-2 positive patients. Secondly, NLR was not an independent prognostic factor in our study. The reason might be the different cut-off value used or different antibody used, also some studies defined >1% as PD-L1 positive cut-off value. In addition, the optimal cut-off values should be verified by other large cohort studies. Thirdly, factors like inflammations or drugs influencing the neutrophil or platelets count were not analyzed in the study, which may have influences on NLR and PLR.
Conclusions
In conclusion, high PLR is significantly associated with poor DFS and OS in breast cancer patients. PD-L1 expression combined with high PLR was significantly associated with an aggressive clinical outcome. Further studies are needed to evaluate the predictive value of PD-L1 and peripheral blood immune markers.
Supplementary
Procedures of PD-L1 immunohistochemical staining
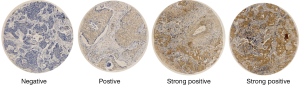
Slides were stained using a Ventana Discovery XT automated system (Ventana Medical Systems, Tucson, AZ, USA) with proprietary reagents according to the manufacturer’s protocol. Briefly, slides were deparaffinized on the automated system with EZ Prep solution (Ventana). A heat-induced antigen retrieval method was used with Cell Conditioning 1 solution (Ventana). The concentration of rabbit primary antibody that reacts to PD-L1 (E1L3N™, Cell Signaling Technology, Beverly, MA, USA) was 1:100 in Dako antibody diluent; slides were incubated with this antibody overnight at 4 °C. Then, the slides were incubated with Ventana Omni Mapanti-rabbit secondary antibody for 60 min. A Ventana Chromo MapKit was used for antibody detection, and then the slides were counterstained with hematoxylin. Next, the slides were dehydrated and cover slipped as per normal laboratory protocol. All slides were independently examined by two pathologists; both of whom had no prior knowledge of the clinical parameters of the patient. Discrepancies were resolved through the simultaneous re-examination of the slides using a double-headed microscope by both pathologists. Figure S5 showed PD-L1expression of tumor cells.
Acknowledgments
We are thankful to the anonymous reviewers for their insightful comments and great efforts to improve the manuscript.
Funding: This work was supported by grants from the National Natural Science Foundation of China (81502302), and Science and Technology Program of Guangdong Province (2014A020212384; 2016A020215079).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.05.39). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Sun Yat-sen Cancer Center Institution Review Board (No. YB2016-068) and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature 2001;411:375-9. [Crossref] [PubMed]
- Spano D, Zollo M. Tumor microenvironment: a main actor in the metastasis process. Clin Exp Metastasis 2012;29:381-95. [Crossref] [PubMed]
- Liu S, Lachapelle J, Leung S, et al. CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast Cancer Res 2012;14:R48. [Crossref] [PubMed]
- Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011;480:480-9. [Crossref] [PubMed]
- Baptista MZ, Sarian LO, Derchain SF, et al. Prognostic significance of PD-L1 and PD-L2 in breast cancer. Hum Pathol 2016;47:78-84. [Crossref] [PubMed]
- Chen S, Wang RX, Liu Y, et al. PD-L1 expression of the residual tumor serves as a prognostic marker in local advanced breast cancer after neoadjuvant chemotherapy. Int J Cancer 2017;140:1384-95. [Crossref] [PubMed]
- Wang C, Zhu H, Zhou Y, et al. Prognostic Value of PD-L1 in Breast Cancer: A Meta-Analysis. Breast J 2017;23:436-43. [Crossref] [PubMed]
- Schalper KA. PD-L1 expression and tumor-infiltrating lymphocytes: Revisiting the antitumor immune response potential in breast cancer. Oncoimmunology 2014;3:e29288 [Crossref] [PubMed]
- Okabe M, Toh U, Iwakuma N, et al. Predictive factors of the tumor immunological microenvironment for long-term follow-up in early stage breast cancer. Cancer Sci 2017;108:81-90. [Crossref] [PubMed]
- Qin T, Zeng YD, Qin G, et al. High PD-L1 expression was associated with poor prognosis in 870 Chinese patients with breast cancer. Oncotarget 2015;6:33972-81. [Crossref] [PubMed]
- Ha H, Nam AR, Bang JH, et al. Soluble programmed death-ligand 1 (sPDL1) and neutrophil-to-lymphocyte ratio (NLR) predicts survival in advanced biliary tract cancer patients treated with palliative chemotherapy. Oncotarget 2016;7:76604-12. [Crossref] [PubMed]
- Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014;106:dju124 [Crossref] [PubMed]
- Proctor MJ, Morrison DS, Talwar D, et al. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer 2011;47:2633-41. [Crossref] [PubMed]
- Kim EY, Lee JW, Yoo HM, et al. The Platelet-to-Lymphocyte Ratio Versus Neutrophil-to-Lymphocyte Ratio: Which is Better as a Prognostic Factor in Gastric Cancer? Ann Surg Oncol 2015;22:4363-70. [Crossref] [PubMed]
- Tang L, Li X, Wang B, et al. Prognostic Value of Neutrophil-to-Lymphocyte Ratio in Localized and Advanced Prostate Cancer: A Systematic Review and Meta-Analysis. PLoS One 2016;11:e0153981 [Crossref] [PubMed]
- Yodying H, Matsuda A, Miyashita M, et al. Prognostic Significance of Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio in Oncologic Outcomes of Esophageal Cancer: A Systematic Review and Meta-analysis. Ann Surg Oncol 2016;23:646-54. [Crossref] [PubMed]
- Zhang H, Gao L, Zhang B, et al. Prognostic value of platelet to lymphocyte ratio in non-small cell lung cancer: a systematic review and meta-analysis. Sci Rep 2016;6:22618. [Crossref] [PubMed]
- Ethier JL, Desautels D, Templeton A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res 2017;19:2. [Crossref] [PubMed]
- Koh CH, Bhoo-Pathy N, Ng KL, et al. Utility of pre-treatment neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as prognostic factors in breast cancer. Br J Cancer 2015;113:150-8. [Crossref] [PubMed]
- Azab B, Shah N, Radbel J, et al. Pretreatment neutrophil/lymphocyte ratio is superior to platelet/lymphocyte ratio as a predictor of long-term mortality in breast cancer patients. Med Oncol 2013;30:432. [Crossref] [PubMed]
- Zhu Y, Si W, Sun Q, et al. Platelet-lymphocyte ratio acts as an indicator of poor prognosis in patients with breast cancer. Oncotarget 2017;8:1023-30. [PubMed]
- Mori H, Kubo M, Yamaguchi R, et al. The combination of PD-L1 expression and decreased tumor-infiltrating lymphocytes is associated with a poor prognosis in triple-negative breast cancer. Oncotarget 2017;8:15584-92. [Crossref] [PubMed]
- Tsang JY, Au WL, Lo KY, et al. PD-L1 expression and tumor infiltrating PD-1+ lymphocytes associated with outcome in HER2+ breast cancer patients. Breast Cancer Res Treat 2017;162:19-30. [Crossref] [PubMed]
- Zhou X, Du Y, Huang Z, et al. Prognostic value of PLR in various cancers: a meta-analysis. PLoS One 2014;9:e101119 [Crossref] [PubMed]
- Templeton AJ, Ace O, McNamara MG, et al. Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2014;23:1204-12. [Crossref] [PubMed]
- Ocana Fernandez A, Templeton AJ, Casas M, et al. Prognostic role for derived neutrophil-to-lymphocyte ratio in early breast cancer. Ann Oncol 2016;27:145O-O.
- Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860-7. [Crossref] [PubMed]
- Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer 2011;11:123-34. [Crossref] [PubMed]
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008;454:436-44. [Crossref] [PubMed]
- Eyileten C, Majchrzak K, Pilch Z, et al. Immune Cells in Cancer Therapy and Drug Delivery. Mediators Inflamm 2016;2016:5230219
- Marzec M, Zhang Q, Goradia A, et al. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1). Proc Natl Acad Sci U S A 2008;105:20852-7. [Crossref] [PubMed]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [Crossref] [PubMed]
- Parsa AT, Waldron JS, Panner A, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med 2007;13:84-8. [Crossref] [PubMed]
- Sabatier R, Finetti P, Mamessier E, et al. Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget 2015;6:5449-64. [Crossref] [PubMed]
- Li F, Ren Y, Wang Z. Programmed death 1 Ligand 1 expression in breast cancer and its association with patients' clinical parameters. J Cancer Res Ther 2018;14:150-4. [Crossref] [PubMed]
- Beckers RK, Selinger CI, Vilain R, et al. Programmed death ligand 1 expression in triple-negative breast cancer is associated with tumour-infiltrating lymphocytes and improved outcome. Histopathology 2016;69:25-34. [Crossref] [PubMed]
- Wimberly H, Brown JR, Schalper K, et al. PD-L1 Expression Correlates with Tumor-Infiltrating Lymphocytes and Response to Neoadjuvant Chemotherapy in Breast Cancer. Cancer Immunol Res 2015;3:326-32. [Crossref] [PubMed]
- Brockhoff G, Seitz S, Weber F, et al. The presence of PD-1 positive tumor infiltrating lymphocytes in triple negative breast cancers is associated with a favorable outcome of disease. Oncotarget 2017;9:6201-12. [Crossref] [PubMed]
- Li Y, Opyrchal M, Yao S, et al. The role of programmed death ligand-1 and tumor-infiltrating lymphocytes in breast cancer overexpressing HER2 gene. Breast Cancer Res Treat 2018;170:293-302. [Crossref] [PubMed]
- Wang C, Sun W, Ye Y, et al. In situ activation of platelets with checkpoint inhibitors for post-surgical cancer immunotherapy. Nature Biomedical Engineering 2017;1:0011.