
Efficient cancer gene therapy with a Del1 fragment administered by hypodermic injection in a mouse explanted tumor model
Introduction
Numerous technologies have been developed to cure cancer patients. However, surgery, chemotherapy and radiation therapy have limited applications for advanced cancer. Surgery and radiation therapy cannot be repeated many times, and cancer cells develop resistance to chemotherapy in many cases. Additionally, the physical conditions of the patients make some therapeutics unusable. Because of these hurdles, the development of novel therapeutic options is required. One such candidate therapeutic option is biological therapy that exerts its effects by exploiting the biological characteristics of cancer cells and their hosts.
Kitano et al. attempted gene therapy in mice with explanted tumors of human squamous cell carcinoma cell line SCCKN using a non-viral vector encoding a fragment of the extracellular matrix protein Del1 (1-3). Plasmid DNA of the fragment was intratumorally injected into explanted tumors, which significantly suppressed tumor growth and improved lifespan. However, restriction of the administration route to intra-tumoral injection limits the applicability of this therapy. If remote administration was effective, it could be an advantage of this therapy. In the present study, we investigated whether hypodermic injection of plasmid cDNA as a non-viral vector was effective against a remotely explanted tumor.
The Del1 protein consists of five domains: three epidermal growth factor repeats (E1, E2, and E3) and two discoidin domains (C1 and C2) (4). E3 induces apoptosis both in vitro and in vivo (5). It also increases gene transfer efficiency (6,7). C1 is essential for deposition of Del1 in the extracellular matrix and can be used as a tool to deposit and concentrate proteins in the extracellular matrix (8,9). In a study by Kitano et al., DNA encoding a recombinant protein consisting of E3 and C1 (referred to as E3C1) was intratumorally injected twice a week. This protocol resulted in efficient deposition of the recombinant protein in the tumor and an increase in gene transfer efficiency compared with a control protein without E3C1. E3C1 gene therapy induced apoptosis and suppressed local invasion of cancer cells. Their study showed that the explanted tumor was larger and grew faster in control mice than in E3C1-treated mice. In contrast, treatment of mice with E3C1 suppressed tumor growth even when the initial tumors were large. Because treatment with 100 µL of an E3C1 DNA solution was effective against tumors of 3,000–5,000 mm3 in volume, it was suggested that E3C1 treatment was effective at a distance from the injection site.
In the present study, we examined the therapeutic efficiency of hypodermic injection using the same recombinant protein from our previous study. This study showed that repeated hypodermic injection of E3C1 cDNA using a non-viral vector significantly suppressed tumor growth and improved lifespan in a mouse tumor explanted model. In addition to the advantages of the safety and low cost of a non-viral vector, the ease of administration shown in this study increases the potential use of cancer therapy employing E3C1.
Methods
Cell line and culture
Human oral squamous cell carcinoma cell line A431 (American Type Culture Collection, Manassas, VA, USA) was employed to investigate whether E3C1 therapy was effective against cancer cells other than SCCKN cells. A431 cells were grown in serum-free 64 medium [60% Opti-MEM (Invitrogen, Carlsbad, CA, USA) and 40% LHC-8 medium (Invitrogen)]. Cells were cultured at 37 °C with 5% CO2.
DNA constructs
Mouse Del1 cDNA was a gift from Dr. Quertermous (Stanford University, Stanford, CA, USA). To construct the plasmid, a fragment encoding E3 and C1 sequences (E3C1, amino acids 122–316 of mouse Del1) was amplified by polymerase chain reaction (PCR) with forward primer 5'-TGTGAAGCTGAGCCTTGCAGAATGGCCGGA and reverse primer 5'-ACAGCCTGAGAGCTCACAGCCAAGAAGTT. The signal peptide sequence of Del1 and E3C1 cDNA was inserted into pcDNA3.1D (Invitrogen). This plasmid is an expression vector with a cytomegalovirus promoter sequence and is designed to add a V5-tag at the C-terminal end of a recombinant protein. To investigate the distribution of E3C1 protein in serum, a plasmid encoding a recombinant fusion E3C1 protein with a heat-stable alkaline phosphatase (AP)-tag was constructed (10,11). Briefly, an E3C1 fragment was generated by reverse transcriptase-PCR and cloned into an AP-tag4 vector (GenHunter, Nashville, TN, USA) for production of AP-tagged E3C1 as a secreted protein. Plasmid DNA without AP or E3C1 was used as the control. Before measurement of serum AP activity, the serum was heated at 68 °C for 60 min to inactivate endogenous AP. The serum AP activity of AP-tagged E3C1 was then measured in a 96-well plate. The enzymatic reaction was initiated by mixing 20 µL conditioned medium and 200 µL substrate [1 mg/mL p-nitrophenyl phosphate (Sigma, St. Louis, MO, USA) in 1 mM MgCl2 and 1 M diethanolamine, pH =9.8] in each well. Absorbance at 405 nm was then measured after 30 min.
Animal experiments
All animal experiments were carried out in accordance with both Nihon University and Japan animal care regulations. This study was approved by the Ethics Committee of Nihon University (AP13M045). Nu/Nu athymic nude mice were kept in a pathogen-free environment. For tumor explants, A431 cells were first seeded in 10-cm culture dishes, cultured overnight to 70–80% confluence, and harvested using a cell stripper (Asone, Tokyo, Japan) after washing with phosphate buffered saline (PBS). Cells were washed twice with PBS, centrifuged at 500 g for 5 min, and then resuspended in 64 medium at 1×107 cells/100 µL. A total of 100 µL suspended cells was injected subcutaneously into the right or both flanks of a mouse. Animals were monitored twice weekly for tumor growth. When tumors were observed, their size was measured in two perpendicular dimensions using calipers. The tumor volume (mm3) was calculated using the formula (width × length2/2). When the tumor volume exceeded 60 mm3, treatment with pE3C1 or pcDNA3, a mock vector, was initiated. For treatment to examine the remote effects of intra-tumoral injection into mice with two explanted tumors, 10 µg DNA was injected into the left tumor in 100 µL in vivo Jet-PEI (PolyPlus-transfection; San Marcos, CA) once a week (mock treatment, n=4; E3C1 treatment, n=4). For treatment to examine the remote effects of hypodermic injection into mice with an explanted tumor, 10 µg DNA was injected into the left flank of a mouse once a week (mock treatment, n=6; E3C1 treatment, n=6). Mice were euthanized when tumors had grown to more than 15% of body weight or on day 60. For histology, after 2 weeks of treatment, mice were sacrificed under deep anesthesia with isoflurane, tumors were harvested, and 5 µm-thick frozen sections were prepared. To investigate the distribution of E3C1 protein in serum, the plasmid encoding the AP-E3C1 fusion protein or control plasmid (pcDNA3) were injected with In vivo Jet-PEI subcutaneously into the right flank of ICR mice (n=4). Forty-eight hours after injection, blood was obtained by cardiac puncture from anesthetized mice.
Immunohistochemistry
A rabbit polyclonal antibody against cleaved caspase-3 (Trevigen, Gaithersburg, MD, USA) and a rabbit monoclonal antibody against CD8 (Abcam) were purchased. An Alexa Fluor 488-labeled goat anti-rabbit antibody and a V5-tag monoclonal antibody conjugated with DyLight 488 were obtained from Invitrogen. For immunohistochemistry, 5 µm-thick sections were fixed with 4% paraformaldehyde, incubated with primary antibodies, and then incubated with the appropriate secondary antibodies. Hoechst 33342 was used to visualize nuclei. An Axioscope 2 (Carl Zeiss Microimaging Inc., Welwyn Garden City, UK) equipped with an AxioCam (Carl Zeiss) was used to observe tissues and acquire images. The number of CD8 positive cells per field was counted using Popimaging software (Digitalbeeing kid, Kanagawa, Japan) (n=4).
Statistical analysis
Results are expressed as means ± standard deviation (SD). Wilcoxon’s rank sum test was performed as appropriate. Statistical significance was defined as P<0.05.
Results
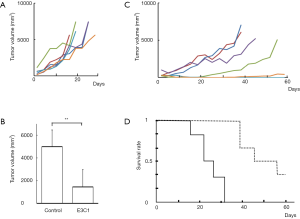
We anticipated that hypodermic injection of E3C1 cDNA would induce adverse effects at the injection site, because it has been reported that intra-tumoral injection of E3C1 cDNA induces apoptosis in the tumor. Therefore, to examine whether injection of E3C1 cDNA had any effect on tumors located far from the injection site, a tumor model mouse with two explanted tumors was established. In this model mouse, one of the tumors was injected with E3C1 cDNA, which was the source of E3C1 protein for the other tumor. The size of the other tumor was measured to evaluate the effect of E3C1 therapy. The growth of E3C1-treated tumors was slower than that of control tumors. On day 10, the tumor volumes in control and E3C1-treated mice were 2,211±1,036 and 1,457±1,054 mm3, respectively (P=0.08). Control mice died from day 12 to 18. In contrast, E3C1 mice died from day 12 to 46. These data suggested that remote injection with E3C1 cDNA affected implanted tumors and could be used instead of intratumoral injection.
Next, the effects of E3C1 treatment by hypodermic injection were examined. The cDNA solution of E3C1 was subcutaneously injected into the back of the mouse at the opposite side to the tumor. Intratumoral injection of E3C1 has been reported to induce apoptosis in the tumor. However, over the observation period in the present study, no obvious adverse effects were found at the injection site (data not shown). Tumor growth in individual mice is shown in Figure 1A (control) and Figure 1B (E3C1). One of the E3C1-treated tumors disappeared, and the growth of E3C1-treated tumors was obviously slower than that of control tumors. After 2 weeks of treatment, control and E3C1-treated tumor volumes were 4,988±1,477 and 1,443±1,523 mm3, respectively (control vs. E3C1, P<0.05) (Figure 1C). Hypodermic injection of E3C1 cDNA significantly suppressed tumor growth and improved lifespan (P<0.05) (Figure 1D). Control mice died or were euthanized within 32 days. In contrast, E3C1-treated mice started to die on day 39, and two of the six mice survived for 60 days at which time the mice were euthanized.
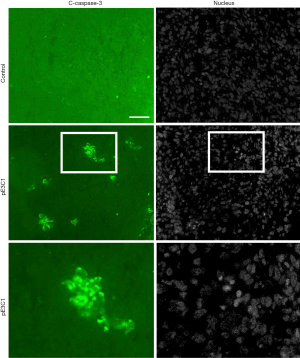
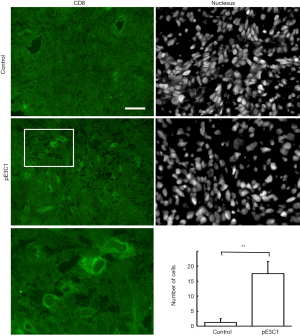
Next, we investigated the mechanism by which hypodermic injection of E3C1 cDNA modulated tumor growth. We first determined whether hypodermic injection of E3C1 cDNA induced apoptosis in tumors as previously observed in tumors that were intratumorally injected with E3C1 cDNA. In control mice, cleaved caspase-3 was not detected in the tumor by immunohistochemistry. Conversely, clusters of caspase-3-positive cells were detected in the tumors of E3C1-treated mice (Figure 2). To examine whether recombinant E3C1 protein encoded by the injected cDNA moved from the hypodermic layer into blood, cDNA encoding heat-stable AP-tagged E3C1 was injected subcutaneously, and then heat-stable AP activity was measured in serum. Heat-stable AP activities in the serum of control and E3C1-treated mice were 85.3±12.3 and 131±39 au, respectively (control vs. E3C1, P<0.05). These data indicated that hypodermic injection of AP-tagged E3C1 cDNA resulted in detectable movement of the recombinant protein into blood. Next, the distribution of recombinant E3C1 used for treatment was examined by immunohistochemistry. The results revealed no recombinant E3C1 in pE3C1-treated samples (data not shown). Moreover, to evaluate remote effects of E3C1 via immunological reactions, we performed immunohistochemistry using an antibody against CD8 (Figure 3). In E3C1-treated tumors, the number of CD8-positive cells was increased to 17.5±4.0 per field from 1.3±1.3 per field in control tumors (P<0.01).
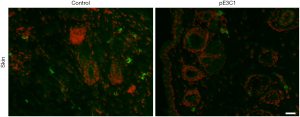
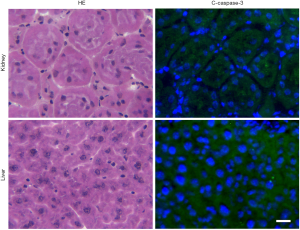
Considering the potential clinical application of this therapy, we investigated the side effects of hypodermic injection of E3C1 cDNA. Because intra-tumoral injection with E3C1 cDNA resulted in cancer cell apoptosis, we determined whether hypodermic injection of E3C1 cDNA induced apoptosis at the injected site in the skin (Figure 4). In injection sites, there were some apoptotic cells that are assumed to be caused by injection. In microscopic observation, no differences were found between control samples and pE3C1 injected samples. Next, potential side effects of E3C1 treatment on other organs, such as the liver and kidneys, were investigated. Apoptosis was not found in the liver or kidneys of either control or E3C1-treated mice (Figure 5).
To examine whether there were any adverse effects of E3C1 treatment on mouse growth, mice without a tumor were injected with E3C1 cDNA for 100 days and their body weights were measured. The body weight of control and E3C1-treated mice increased by 1.26±0.07 and 1.27±0.07 times, respectively, during the 100 days, indicating no significant change in the growth of E3C1-treated mice.
Discussion
This study showed that gene therapy with E3C1 cDNA using a non-viral vector was effective against tumors of explanted A431 cells in mice, even when the plasmid DNA was administered by hypodermic injection. Such administration induced apoptosis of tumor cells and suppressed tumor growth, resulting in improvement of lifespan.
In this study, to deposit the recombinant protein in the extracellular matrix, the C1 domain of Del1 was fused with its E3 domain, which has a cytotoxic activity. However, following injection into the skin, the exogenous E3C1 recombinant protein tagged with AP was detected in blood. This phenomenon may be due to gradual release of recombinant protein with the C1 domain from the extracellular matrix (8).
There are two possible mechanisms to explain the remote effects of hypodermically injected E3C1 gene transfer. One mechanism is a systemic reaction, such as tumor immunity, which suppresses tumor growth. Thus, some immunomodulating cytokines, such as interferon alpha, 2 beta, and gamma, can be administered subcutaneously and intramuscularly for treatment of malignant diseases (12,13). The number of CD8-positive cells was increased by E3C1 treatment in this study. Therefore, an immunological mechanism may be involved in E3C1 treatment. Because the recombinant E3C1 was not detected in explanted tumors, E3C1 possibly serves a role in the immunological system in a systemic manner.
The other mechanism is transport of the fusion protein via the bloodstream from the injection site to the tumor. It was expected that hypodermic injection of E3C1 cDNA would induce apoptosis at the injection site. However, no obvious apoptosis was found. Additionally, no significant apoptosis was found in other major organs. This study suggests that the absence of adverse effects on other tissues were not caused by localized distribution of E3C1 at its injection site, but likely due to the effects of EC31 being tumor specific. Tumor-specific effects of E3C1 can be discussed from the viewpoint of Del1 functions.
Del1 is expressed in embryonic endothelial cells and in the early embryo where vascular formation is active (4). Overexpression of Del1 enhances vascular remodeling and decreases total vessel length in the mesenterium (14). However, the Del1 domains responsible for these effects have not yet been determined. Additionally, Aoka et al. reported that vasculatures in some kinds of tumors express Del1 (15). Because embryos and tumors share the characteristics of active angiogenesis and remodeling, tumor-specific effects of E3C1 might result from these characteristics. Blood vessels in cancers have been reported to have specific characteristics (16,17). Most notably, they show highly active growth which is why an anti-vascular endothelial growth factor antibody is an effective therapeutic agent against some kinds of cancer (18,19). Another feature of blood vessels in cancer is that the phospholipid phosphatidylserine is exposed on the endothelial cell surface. This feature is in contrast to normal cells in which phosphatidylserine is distributed to the inner leaflet of the plasma membrane. Currently, an anti-phosphatidylserine antibody that inhibits angiogenesis in cancer is undergoing clinical trials (20). These anti-angiogenesis agents have tumor-specific effects even when administered systemically. To clarify the mechanism of the tumor-specific effect of E3C1, the effect of E3C1 treatment on tumor vessels should be analyzed.
The limitation of E3C1 cancer therapy is that it is not eradicative. The explanted tumor disappeared in just one of six mice. Therefore, combinatorial therapy with other therapeutic methods may be beneficial. E3C1 cancer therapy is effective, low cost, and simple. Qualification of the therapy is the next milestone. Mamiya et al. reported that intravenous injection with a recombinant E3 domain results in sudden death of mice (7). The toxicity and usage of the E3 domain should therefore be studied further.
Acknowledgments
Mouse Del1 cDNA was a kind gift from Dr. Quertermous (Stanford University, Stanford, CA, USA). We thank Forte Inc. for English editing. We also thank Mitchell Arico from Edanz Group (
Funding: This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education and Culture of Japan (22792019).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.05.45). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All animal experiments were carried out in accordance with both Nihon University and Japan animal care regulations. This study was approved by the Ethics Committee of Nihon University (AP13M045).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kitano H, Mamiya A, Kokubun S, et al. Efficient nonviral gene therapy with FasL and Del1 fragments in mice. J Gene Med 2012;14:642-50. [Crossref] [PubMed]
- Kitano H, Atsushi Mamiya, Hidai C. Improvement of FasL gene therapy in vitro by fusing the FasL to Del1 protein domains. Targets in Gene Therapy 2011. Available online: https://www.intechopen.com/books/targets-in-gene-therapy/improvement-of-fasl-gene-therapy-in-vitro-by-fusing-the-fasl-to-del1-protein-domains
- Abrami L, Liu S, Cosson P, et al. Anthrax toxin triggers endocytosis of its receptor via a lipid raft-mediated clathrin-dependent process. J Cell Biol 2003;160:321-8. [Crossref] [PubMed]
- Hidai C, Zupancic T, Penta K, et al. Cloning and characterization of developmental endothelial locus-1: an embryonic endothelial cell protein that binds the alphavbeta3 integrin receptor. Genes Dev 1998;12:21-33. [Crossref] [PubMed]
- Kitano H, Kokubun S, Hidai C. The extracellular matrix protein Del1 induces apoptosis via its epidermal growth factor motif. Biochem Biophys Res Commun 2010;393:757-61. [Crossref] [PubMed]
- Kitano H, Hidai C, Kawana M, et al. An epidermal growth factor-like repeat of Del1 protein increases the efficiency of gene transfer in vitro. Mol Biotechnol 2008;39:179-85. [Crossref] [PubMed]
- Mamiya A, Kitano H, Takao K, et al. An Epidermal Growth Factor Motif from Del1 Protein Increases the Efficiency of In Vivo Gene Transfer with a Non-Viral Vector. Mol Biotechnol 2013;54:445-50. [Crossref] [PubMed]
- Hidai C, Kawana M, Kitano H, et al. Discoidin domain of Del1 protein contributes to its deposition in the extracellular matrix. Cell Tissue Res 2007;330:83-95. [Crossref] [PubMed]
- Hidai C, Kitano H, Kokubun S. The Del1 deposition domain can immobilize 3alpha-hydroxysteroid dehydrogenase in the extracellular matrix without interfering with enzymatic activity. Bioprocess Biosyst Eng 2009;32:569-73. [Crossref] [PubMed]
- Lowe ME. Site-specific mutations in the COOH-terminus of placental alkaline phosphatase: a single amino acid change converts a phosphatidylinositol-glycan-anchored protein to a secreted protein. J Cell Biol 1992;116:799-807. [Crossref] [PubMed]
- Berger J, Hauber J, Hauber R, et al. Secreted placental alkaline phosphatase: a powerful new quantitative indicator of gene expression in eukaryotic cells. Gene 1988;66:1-10. [Crossref] [PubMed]
- Miller CH, Maher SG, Young HA. Clinical Use of Interferon-gamma. Ann N Y Acad Sci 2009;1182:69-79. [Crossref] [PubMed]
- Negrier S, Escudier B, Lasset C, et al. Recombinant human interleukin-2, recombinant human interferon alfa-2a, or both in metastatic renal-cell carcinoma. Groupe Francais d'Immunotherapie. N Engl J Med 1998;338:1272-8. [Crossref] [PubMed]
- Hidai C, Kawana M, Habu K, et al. Overexpression of the Del1 gene causes dendritic branching in the mouse mesentery. Anat Rec A Discov Mol Cell Evol Biol 2005;287:1165-75. [Crossref] [PubMed]
- Aoka Y, Johnson FL, Penta K, et al. The embryonic angiogenic factor Del1 accelerates tumor growth by enhancing vascular formation. Microvasc Res 2002;64:148-61. [Crossref] [PubMed]
- McDonald DM, Choyke PL. Imaging of angiogenesis: from microscope to clinic. Nat Med 2003;9:713-25. [Crossref] [PubMed]
- Akino T, Hida K, Hida Y, et al. Cytogenetic abnormalities of tumor-associated endothelial cells in human malignant tumors. Am J Pathol 2009;175:2657-67. [Crossref] [PubMed]
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542-50. [Crossref] [PubMed]
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335-42. [Crossref] [PubMed]
- Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov 2015;14:561-84. [Crossref] [PubMed]


