
Androgen deprivation therapy and radiation therapy for prostate cancer: the mechanism underlying therapeutic synergy
Clinical development of androgen deprivation therapy (ADT) for prostate cancer
Metastatic prostate cancer
The first report of modulation of androgens in men with prostate cancer was conducted by Huggins and Hodges in 1941 (1). In a cohort of 8 men with metastatic prostate cancer including osseous metastases, administration of estradiol and/or surgical castration resulted in a durable reduction of alkaline phosphate levels, a marker of osseous disease burden. By surgically eliminating testicular production of testosterone or negatively regulating the hypothalamic-pituitary-gonadal axis through estrogen administration, this was the first demonstration that androgen suppression can induce a measurable biochemical disease response.
Though exogenous estrogen proved to be an effective non-surgical strategy to reduce circulating testosterone to near-castrate levels, it was ultimately found to cause significant toxicity including—but not limited to—endocrine and metabolic changes (weight gain, hot flashes, gynecomastia), cardiovascular toxicity (edema, hypertension, stroke, thromboembolic disease, etc.), arthralgias and mood disorders. In the 1960–1970s, The Veterans Administration and others (2,3) first published data demonstrating that the delivery of estrogen in men with advanced prostate cancer significantly increased the incidence of cardiovascular morbidity and mortality, effecting upwards of 60% of treated men. Concerned that the risks outweighed the benefits, in the 1980s great efforts were made to identify a reversible method to reduce testosterone to castrate levels with less risk to the patient.
Gonadotropin-releasing hormone (GnRH) was first isolated in 1971 (4). Initially appreciated for its ability to stimulate the pituitary to produce luteinizing hormone (LH) and follicle stimulating hormone (FSH), it was investigated for use as a pro-fertility drug in women or in men with hypogonadotropic hypogonadism (5). Once it was recognized that chronic elevation of GnRH paradoxically suppresses pituitary release LH and FSH—and hence testicular production of testosterone—the use of exogenous GnRH was explored for male contraception and precocious puberty.
In 1981, Redding sought to leverage the anti-androgenic effects of GnRH agonists in the context of hormone-sensitive prostate cancer. He administered synthetic GnRH agonists to rats inoculated with two hormone-sensitive prostate cancer cell lines. Daily administration of the GnRH agonist resulted in a significant reduction in prostate tumor volume in the setting of measurable suppression of LH, FSH and testosterone (6).
Encouraged by promising pre-clinical data, in 1983 Wenderoth tested a GnRH agonist, buserelin, in 12 men with advanced prostate cancer. In addition to demonstrating a robust endocrine response—an initial rise followed by reduction in LH, FSH and testosterone to near castrate levels—patients with osseous metastases experienced durable relief from bone pain while others were found to have significant regression of soft tissue disease and improvement in performance status (7).
By the mid-1980s synthetic GnRH agonists were being developed for clinical use in patients with advanced prostate cancer, including goserelin (Zoladex) depot formulations (8) and leuprolide (Lupron) (9)—both still used today.
Seeking more potent forms of ADT to improve disease control for patients with metastatic prostate cancer, a GnRH agonist, leuprolide, was combined with a nonsteroidal androgen receptor (AR) antagonist, flutamide, designed to block binding of residual circulating testosterone to AR. The combination therapy was found to increase progression-free survival and median survival, while preventing initial osseous pain flairs (10). Hence the GnRH agonist and AR antagonist combination therapy became the preferred form of ADT.
Locally advanced prostate cancer
In the 1980s, patients with locally advanced prostate cancer were treated definitively with radiation therapy. However, the risk of locoregional failures after radiation therapy was known to increase with higher initial disease burden (11). With the success of ADT in the metastatic setting, the Radiation Therapy Oncology Group (RTOG) initiated a series of trials to evaluate the potential benefits of ‘cytoreductive’ ADT delivered in combination with definitive radiation therapy in an effort to improve disease control.
In 1983, RTOG launched a phase II clinical trial, RTOG 83-07, to evaluate the clinical effectiveness and potential toxicity of diethylstilbestrol (DES), a synthetic nonsteroidal estrogen, versus megestrol, a steroidal progestin, in prostate cancer patients with organ-confined disease or extension beyond the prostate (T2/T3). DES proved to more toxic than megestrol with comparable rates of local failure (12).
Hence, a second phase II clinical trial was initiated by RTOG to evaluate a potentially more potent and less toxic approach. RTOG 85-19 evaluated the cytoreductive and possible synergistic role of combined GnRH agonist, goserelin, plus the non-steroidal anti-androgen, flutamide, with concurrent definitive radiation therapy for patients with locally advanced disease (T2–3, N0–1). ADT was delivered 2 months prior to, and for the duration of radiation therapy. This study demonstrated that the combination was well tolerated, successfully suppressed testosterone to castrate levels, and resulted in regression of palpable prostate tumors in 93% (28/30) patients (13).
The promising results of RTOG 85-19 led to the initiation of a phase III randomized trial, RTOG 86-10, including patients with bulky (≥25 cm), locally advanced prostate cancer (T2–4, N0–1). 417 patients were randomized to ADT (goserelin, flutamide) 2 months prior to and concurrently with definitive radiation therapy, versus radiation therapy alone. At 8 years, short term-ADT improved local control, biochemical disease-free survival, reduced the incidence of distant metastases and cause-specific mortality, but did not significantly improve overall survival. Subset analysis suggested that patients with lower Gleason score (GS 2–6) benefited most, demonstrating an overall survival benefit—70% versus 52% (14).
The authors of RTOG 85-19 noted that “the mechanism of interaction between radiation therapy and androgen deprivation in carcinoma of the prostate remain largely unknown.” They suggested that in addition to reducing tumor volume, when offered concurrently with radiation therapy, ADT “may interact with radiation on a cellular level.” (14).
Meanwhile, the role of long-term ADT in the adjuvant setting was evaluated in RTOG 85-31. Patients with high-risk locally advanced disease (cT3 N0; T1–2 N1) or those with positive margins (R1) or seminal vesical invasion (pT3b) after surgery were randomized to definitive radiation therapy followed by adjuvant ADT (goserelin) or radiation therapy alone. Adjuvant ADT was initiated immediately after radiation therapy and continued indefinitely or until signs of clinical progression. For patients treated with radiation therapy alone, ADT was allowed in the setting of clinically apparent disease recurrence (15). The group treated with immediate adjuvant, long-term ADT demonstrated improvement in overall survival, disease-free control, local control, rate of distant metastases and cause-specific mortality. Notably, the population benefited the most was patients with higher Gleason’s score (8–10>7>2–6).
The RTOG trials initiated in the 1980s taught us that whether from cytoreduction and/or synergy with radiation therapy, short-term ADT delivered in the neoadjuvant and concurrent setting with definitive radiation therapy improves outcomes for patients with locally advanced prostate cancer (RTOG 86-10). Whereas, long-term ADT delivered in the adjuvant setting improved outcomes for patients with high-risk locally advanced disease, especially for patients with a high Gleason’s score (≥8) (RTOG 85-31). Though the clinical benefit of ADT was proven, the optimal timing and duration of ADT for patients with locally advanced prostate cancer remained unanswered.
To address the question of timing and duration of ADT for locally advanced prostate cancer, RTOG 92-02 was initiated. Patients were randomized to neoadjuvant short-term ADT (4 months goserelin plus flutamide) followed by radiation therapy alone versus long-term ADT, neoadjuvant ADT followed by an additional 24 months of goserelin, in combination with radiation therapy. At 10 years, long-term ADT improved all endpoints, compared to short-term ADT, including disease-free survival, disease-specific survival, local progression, distant metastases and biochemical failure—except overall survival. However, in subset analysis, patient with Gleason’s score 8–10 achieved an overall survival benefit (16).
Similarly, the EORTC 22863 conducted a phase III clinical trial evaluating the role of long-term ADT (1 month cyproterone, goserelin for 3 years) combined with definitive radiation therapy versus definitive radiation therapy alone in men with high-risk prostate cancer (T1–2, G3, N0–1;T 3–4, G1–3, N0–1). At 10 years, locoregional control, disease-free survival, overall survival, distant metastases-free survival and overall survival were all improved (17).
Together, RTOG 92-02 and EORTC 22863 demonstrated a clinically meaningful benefit of long-term ADT delivered concurrently with definitive radiation therapy for patients with locally advanced, high-risk disease, which remains the standard of care (18).
Localized, intermediate risk prostate cancer
Proven effective for metastatic and high-risk, locally advanced prostate cancer, the use of ADT in combination with definitive radiation therapy was extended to patients with intermediate risk disease. Because survival in these patients is years to decades, short-term ADT was evaluated in these patients, rather than long-term ADT, in order to optimize the risk-benefit ratio by minimizing unwanted acute and chronic toxicities.
The role of ADT combined with definitive radiation therapy for intermediate risk prostate cancer was evaluated in three landmark clinical trials. RTOG 94-08 demonstrated that for patients with low- and intermediate-risk prostate cancer, short term ADT (4 months) improved outcomes at 10 years, with the largest benefit in the intermediate risk group (19). While TROG 96.01 confirmed that 6 months of ADT was superior to 3 months of ADT for patients with intermediate and high-risk prostate cancer (20). Focusing on the unfavorable intermediate risk group (cT1b-cT2b N0; with >1: PSA >10, Gleason’s score 7–10, cT3), D’Amico demonstrated that 6 months of ADT improved overall survival, prostate cancer-specific mortality and all-cause mortality (21). However, the 15-year follow-up suggested that in the subset of patients with moderate-to-severe comorbidities, the addition of ADT significantly increased overall mortality and cardiac mortality (22).
Today, patients with unfavorable intermediate risk prostate cancer treated with definitive radiation therapy are recommended to complete a short-term ADT (4–6 months) in the neoadjuvant and concurrent setting, comorbidities permitting (18).
Preclinical evidence for synergy between ADT and radiation therapy
Cellular mechanism of action
While ADT was being developed clinically for use in patients with prostate cancer, others were investigating the mechanism by which suppression of androgens improves disease control. There was conjecture about the clinical benefit of ADT being a result of cytoreduction or debulking of disease prior to radiation therapy, while others surmised a synergy between ADT and radiation therapy at the cellular level.
In men, the majority of circulating testosterone is produced by leydig cells in the testes following stimulatory signals produced by the pituitary, LH and FSH. The remainder of testosterone is produced by the adrenal gland, derived from circulating steroid hormone precursors (DHEA and androstenedione). Once produced, testosterone is transported protein-bound to the target cell where it is metabolized intracellularly by 5α-reductase to the super-active metabolite, dihydrotestosterone (DHT). DHT binds its cognate receptor, the AR, which subsequently dimerizes and is translocates into the nucleus where it conducts its DNA-directed functions, including transcriptional regulation of target genes (23).
Different forms of ADT suppress androgen signaling through a variety of mechanisms: inhibition of the hypothalamic-pituitary-gonadal axis (GnRH agonists, e.g., leuprolide), inhibition of steroid hormone biogenesis in the testes and adrenal glands (17α-hydroxylase, e.g., abiraterone), inhibition of conversion of testosterone to DHT (5α-reductase, e.g., finasteride) and blockade of androgen binding to the AR (AR antagonists, enzalutamide). Together, these therapies obstruct AR-driven transcription, fundamentally altering the transcriptional program of the cell.
As early as 1966, it was recognized that RNA biosynthesis in both the normal and malignant prostate is androgen-dependent, suggesting that the hormonal milieu is capable of altering cellular metabolism of a prostate cell (24). Later, Burges demonstrated that surgical castration of normal male rats dramatically upregulated the rate of cellular apoptosis in the prostate. By 48 hours post-castration, the rate of apoptosis of prostate epithelial cells rose from 1% to 20% per day—with a concomitant reduction in cells entering S-phase of the cell cycle, instead entering the quiescent G0 phase (25). These data demonstrate that withdraw of androgens alone significantly downregulates cellular growth and induces cellular death of the seemingly androgen-dependent cells.
The critical importance of AR-signaling for prostate cancer growth is further highlighted in the context of “castrate resistant prostate cancer,” prostate cancer that grows despite androgen levels being suppressed to castrate levels. In these patients, exogenous selection pressure stimulates resistance to ADT through multiple pathways.
In 1995, Visakorpi conducted fluorescence in situ hybridization (FISH) analysis of the AR gene in prostate biopsy samples from patients before and after they developed progression of disease while on ADT. The investigators discovered that 30% of patients who had clinical progression of disease developed amplification of the AR gene, significantly increasing the mean copy number to 3.8–21.5 per cell, which was not present in the corresponding pre-ADT biopsies. The apparent selection pressure of ADT was further suggested by evidence that none of the ADT-naïve patients demonstrated AR gene amplification (26). Amplification of the AR receptor is now commonly referred to as the hypersensitivity resistance pathway, as increasing the number of available AR receptors re-sensitizes the cell to androgen-AR binding. Others have shown that ADT refractory patients may also develop somatic activating mutations of AR enabling binding and activation of the receptor by other naturally existing steroid hormones, now known as the precocious resistance pathway (27).
Together, these data highlight the dependence of prostate cancer on AR signaling and the initial power of therapeutic androgen blockade to alter the transcriptional profile of the cell and hence cellular behavior.
Synergy between radiation therapy and ADT
The clinical trial data from the 1980s confirmed that the addition of ADT to radiation therapy for patients with locally advanced disease improves outcomes. Though initially hypothesized to offer benefit from upfront cytoreduction or reduction in disease burden, others began testing the hypothesis that ADT and radiation therapy may indeed behave in a synergistic fashion.
Using a rat prostate cancer model, Zietman quantified the radiation dose required to eradicate 50% of the tumors (TCD 50) in tumor-bearing rats, before and after orchiectomy. For rats treated with radiation therapy with intact testes, the TCD 50 was 89 Gy. However, in rats who underwent orchiectomy 24 hours versus 12 days (after maximal tumor regression) prior to radiation therapy, the TCD 50 was significantly reduced to 60 and 42.1 Gy, respectively. The authors hypothesized that the significant improvement in local control following orchiectomy may indeed be attributed to cytoreduction yielding fewer malignant cells to target with radiation therapy and perhaps improved oxygenation of smaller tumors. On the other hand, the authors proposed that orchiectomy and radiation therapy may behave synergistically by further enhancing cellular apoptosis through re-assortment of cells in the cell cycle for synchronization and optimization of radiation-induced damage (28). This seminal study lead the way for others to investigate a possible synergistic relationship between ADT and radiation therapy.
Radiation therapy and DNA damage
Radiation therapy is known to exert its therapeutic effects through DNA damage, namely double-stranded DNA breaks (DSBs). Depending on the phase of the cell cycle, DSBs are repaired by one of two pathways in the DNA damage response (DDR). Non-homologous end joining (NHEJ) is the predominant repair mechanism in G1 phase of the cell cycle, before the mitosis and chromosomal replication has occurred. Whereas homologous recombination (HR) is the main repair mechanism in S/G2, after the chromosomes have been replicated and a sister chromatid is available to serve as a complementary template for repair (Figure 1) (29).
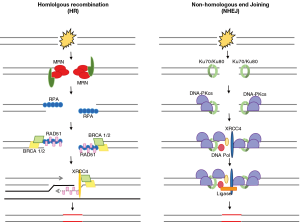
Dysregulation of cell-cycle checkpoint proteins or dysfunctional DDR contributes to genomic instability and plays a role in tumorigenesis through the accumulation of unrepaired mutations. On the other hand, it has been shown that in malignancy, enhanced DDR correlates with radioresistance and progression of disease.
Prostate biopsy samples were examined from men with prostate cancer prior to definitive radiation therapy, in the absence of ADT. Investigators found that the nuclear localization of the catalytic subunit of DNA-PK complex (DNA-PKcs), a key protein kinase complex in the NHEJ repair pathway, strongly correlated to biochemical recurrence. These data suggest that expression and nuclear localization of NHEJ proteins may render a cell equipped for efficient repair of radiation-induced DSBs and thus confer radioresistance (30). Others have shown that inactivating mutations of the NHEJ protein, Ku80—product of the XRCC5 gene and member of the DNA-PK complex—renders cells more sensitive to ionizing radiation (31). These data further support the hypothesis that reduced activity of NHEJ proteins may hinder repair of radiation-induced DSBs and thus increase susceptibility to radiation-induced cell death.
AR and the NHEJ pathway
Early work by Mayeur characterized the composition of the AR protein complex enabling AR-driven transcription using tandem mass spectroscopy. The analysis implicated the NHEJ pathway DNA-PK complex composed of (I) the catalytic subunit, DNA-PKcs, (II) Ku70 and (III) Ku80 as key regulators of AR. The investigators demonstrated that Ku70 and Ku80 bind AR directly at its ligand binding domain (LBD), while all three complex proteins, DNA-PKcs, Ku70, and Ku80 behave as co-activators to enhance AR transcriptional activity. Further, it was shown that Ku70 and Ku80 bind directly to PSA promoter and enhancer elements of the DNA in an androgen-dependent fashion (32). These data implicate the NHEJ pathway DNA-PK complex proteins, DNA-PKcs, Ku70, and Ku80, as integral co-activators promoting effective androgen signaling.
Intrigued by the apparent associated between AR and DNA repair, Polkinghorn (33) conducted a transcriptome analysis of 14 human prostate cancer tissue samples, identifying an AR-associated gene signature comprised of 144 genes involved in the DNA repair response. The authors refined this DNA repair gene signature in vitro, though RNA-seq and ChIP-seq experiments, demonstrating androgen induced AR-binding of the promoter or enhancer region of 32 genes involved in DNA repair, among them the NHEJ proteins, Ku80 and XRCC4. These data add support for AR is a direct regulator of DNA repair pathways, including NHEJ pathway.
Moving beyond transcriptome analyses, the authors investigated the role androgen signaling and ionizing radiation on the production of DNA damage in prostate cancer cells in vitro. When prostate cancer cells were exposed to ADT alone, in the absence of genotoxic ionizing radiation, the ADT-treated cells demonstrated a significant increased DSBs compared to untreated cells, further supporting the hypothesis that DSB repair is, in part, AR-dependent. The combination of ionizing radiation and conditions of androgen depletion resulted in a delayed, but increase in DSBs, compared to cells exposed to androgen. While, the combination ADT and radiation resulted in a significant decreased the clonogenic survival of the prostate cancer cells, independent of distribution of cells in the cell cycle, compared to radiation alone. These data demonstrate that when combined, androgen deprivation radiation therapy more effectively induces and prolongs the existence un-repaired DSB, compared to either therapy alone. To further dissect the mechanism by which DSB repair was compromised by interference in AR signaling, the investigators confirmed functional impairment in the NHEJ DNA repair pathway, but not the HR DNA repair pathway, in ADT-treated cells (33). Together, these data suggest a functional synergy between ADT and radiation therapy through impaired AR-induced NHEJ repair of DNA damage.
Extending the association between DNA damage and repair (DDR) to disease control in vivo, Evans (34) pooled prostatectomy samples from 4 published cohorts including 1,090 men with high-risk prostate cancer. The authors created a DNA damage and repair pathway signature through unsupervised hierarchical clustering of patient-level transcriptome data. The investigators demonstrated a strong correlation between AR expression and DNA repair pathways, consistent with known AR-driven upregulation of DDR pathways. The DDR pathway signature was found to offer prognostic value, predicting improved biochemical-recurrence free survival, metastasis-free survival and overall survival. These data suggest a correlation between expression of DNA damage and repair pathways and improved disease outcomes, consistent with earlier data demonstrating that increase nuclear DNA-PKcs in prostate biopsied positively correlated disease recurrence after radiation therapy (30).
Having demonstrated a correlation between AR-signaling and DNA repair, Al-Ubaidi (35) sought to validate this in vivo, testing the hypothesis that inhibition of AR signaling through surgical or chemical (GnRH agonist) castration impairs DSB repair by the NHEJ pathway. Patients with newly diagnosed locally advanced prostate cancer underwent biopsy followed by treatment with surgical castration or ADT. One month after surgical castration or 2 months after initiation of ADT, patients underwent a second biopsy. The authors demonstrated a 50% reduction the levels of Ku70 both in the nucleus and cytoplasm after castration or ADT. The post-treatment absolute reduction and kinetics of reduction of Ku70 correlated with a reduction in PSA, a known product of AR-driven transcription. Finally, the investigators demonstrated that the decrease in Ku70 levels post-castration correlated with the increase in gamma-H2AX foci, a marker of DNA DSBs, in post-castration biopsies. These data implicate AR as a regulator of the NHEJ pathway protein, Ku70.
Combining the observation that functionally defective NHEJ pathway proteins alter the radiosensitivity of a tissue (30,31) and that ADT or castration significantly depletes nuclear and cytoplasmic Ku70 (35), Tarish (36) tested the ability of neoadjuvant ADT to decrease DNA repair by the NHEJ pathway following radiation therapy.
The authors enrolled 48 patients with localized prostate cancer. All patients underwent a pre-treatment biopsy. Patients were randomized to receive (arm 1) neoadjuvant ADT (GnRH agonist) for 8 weeks versus (arm 2) radiation therapy (2 Gy ×5 fractions) followed by a second biopsy. After the second biopsy, patients’ treatment was reversed: those exposed to ADT (arm 1) were treated with radiation therapy (2Gy ×5 fractions) whereas patients initially treated with radiation therapy (arm 2) were treated with 8 weeks of ADT followed by a third biopsy for all patients. Afterwards, both groups completed definitive radiation therapy. As was shown previously, nuclear Ku70 and AR decreased following ADT, with a statistically significant correlation between nuclear Ku70 and AR. Whereas in the absence of ADT, nuclear Ku70 actually increased following radiation therapy, suggesting radiation-induced upregulation of the NHEJ pathway to repair DSBs (36).
To evaluate the effect of ADT combined with radiation therapy on DSBs, 3 hours after completion of radiation therapy, biopsies were completed and stained with 53BP1 and γ-H2AX—makers of DSBs. Following radiation therapy alone, there was a statically significant increase in both 53BP1 and γ-H2AX foci. However, the number of γ-H2AX-foci, and hence potentially toxic DSB foci, was significantly higher in patients treated with neoadjuvant ADT followed by radiation therapy, compared to radiation therapy alone (36), confirming synergy between ADT and radiation therapy.
To investigate the role of NHEJ enzyme complex in radiation-induced DSB repair, the authors quantified the phosphorylated, active form of the catalytic subunit of DNA-PK (P-DNA-PKcs), demonstrating a statistically significant increase in nuclear P-DNA-PKcs following radiation therapy alone, while patients treated with neoadjuvant ADT followed by radiation therapy demonstrated no change in nuclear P-DNA-PKcs, compared to baseline (36). These data offer further support that ADT blocks radiation-induced activation of the NHEJ pathway typically necessary for maximal DSB repair. Taken together, neoadjuvant ADT functionally impairs the NHEJ pathway, thus decreasing the prostate cancer cell’s ability to repair radiation-induced DNA damage.
Goodwin sought to further elucidate the mechanism by which AR mediates radiation-induced DNA damage repair in prostate cancer (37). The authors demonstrated that ionizing radiation and doxorubicin (genotoxic chemotherapy), both known to induce DSBs—but not UV radiation—induced expression of AR target genes in a dose-dependent fashion, suggesting DSBs are inducers of AR activity.
On the other hand, the AR-dependence of DSB repair was demonstrated when cells treated with ADT and radiation showed a prolongation (>72 vs. 22 hours) of unrepaired DSBs (γ-H2AX and 53BP1 foci), compared to cells treated with radiation alone, suggesting that reduced AR signaling resulted in significantly delayed and incomplete repair of radiation-induced DSBs (37). Further supporting AR-regulation of DNA repair, transcriptome and CHiP-seq analysis confirmed radiation-induced AR binding of DNA damage repair genes, including PRKDC (DNA-PKCcs) and XRCC2 and XRCC3, members of the Rad51 protein family involved in the HR DNA repair pathway.
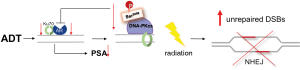
Finally, authors demonstrated a functional association between AR activity and DSB repair though decreased specific activity of DNA-PKcs in vitro following ADT, that was successfully rescued with supplemental androgen. As anticipated, knock-down of DNA-PKcs reduced effective DSB repair. Interestingly, knock-down DNA-PKcs was also found to decrease expression of AR-target genes. Together, these data support the hypothesis that radiation-induced DSB repair is mediated by AR-induced DNA-PKcs activation. On the other hand, DNA-PKcs serves as co-activator of AR (37), consistent with earlier work from Mayeur (32). Hence, the authors proposed a model by which AR is a transcriptional regulator of the NHEJ pathway protein, including DNA-PKcs, that, in turn, serves as a co-regulator of AR, participating in a positive feedback loop to enable efficient repair of DSBs, in the presence of androgen.
The positive feedback loop goes awry with effective inhibition of AR through ADT. In the setting of ADT, AR is down-regulated, which in turn, decreases the abundance of activated DNA-PKcs necessary for NHEJ DNA repair. Reduced DNA-PKcs interrupts the positive feedback through failure to behave as co-activator for AR (Figure 2). It is through interruption of the AR-NHEJ positive feedback that ADT effectively synergizes with radiation therapy to disable the cell’s innate DNA repair machinery, yielding malignant cells with increased sensitivity to radiation-induced DNA damage. As such, ADT effectively behaves as a radiation sensitizer, rendering prostate cells more sensitive to the damaging effects of radiation therapy.
Conclusions
Here, we describe the clinical development of ADT for the treatment of prostate cancer. First described as a palliative therapy in the metastatic setting by Huggins and Hodges in 1941 (1), it is now the standard of care for patients with unfavorable, intermediate risk localized prostate cancer, locally advanced, recurrent prostate cancer and metastatic prostate cancer, in combination with definitive radiation therapy, for non-metastatic patients (18). Though initially thought to be efficacious through cytoreduction and de-bulking of disease, there is a growing body of pre-clinical and clinical data to suggest that ADT and radiation therapy behave synergistically at the cellular level. Here, we have outlined the mechanism by which ADT results in down-regulation of AR-driven repair of DNA double-stranded breaks by the NHEJ pathway, hence serving as a radiation sensitizer. Of critical importance to the clinical practice of Oncology, these data offer mechanistic insight underscoring the importance of neoadjuvant and concurrent ADT in patients with locally advanced prostate cancer undergoing definitive radiation therapy. Finally, the developing mechanistic insight into androgen signaling and DNA repair may offer an opportunity to develop targeted therapies to further improve prostate cancer treatment.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Israel Deutsch, James McKiernan, Charles Drake) for the series “Prostate Cancer: Current Understanding and Future Directions” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.05.42). The series “Prostate Cancer: Current Understanding and Future Directions” was commissioned by the editorial office without any funding or sponsorship. The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J Urol 2002;167:948-51; discussion 952. [Crossref] [PubMed]
- Blackard CE. The Veterans' Administration Cooperative Urological Research Group studies of carcinoma of the prostate: a review. Cancer Chemother Rep 1975;59:225-7. [PubMed]
- Jacobi GH, Altwein JE, Kurth KH, et al. Treatment of advanced prostatic cancer with parenteral cyproterone acetate: a phase III randomised trial. Br J Urol 1980;52:208-15. [Crossref] [PubMed]
- Schally AV, Arimura A, Baba Y, et al. Isolation and properties of the FSH and LH-releasing hormone. Biochem Biophys Res Commun 1971;43:393-9. [Crossref] [PubMed]
- Happ J, Hartmann U, Weber T, et al. Gonadotropin and testosterone secretion in normal human males after stimulation with gonadotropin-releasing hormone (GnRH) or potent GnRH analogs using different modes of application. Fertil Steril 1978;30:666-73. [Crossref] [PubMed]
- Redding TW, Schally AV. Inhibition of prostate tumor growth in two rat models by chronic administration of D-Trp6 analogue of luteinizing hormone-releasing hormone. Proc Natl Acad Sci U S A 1981;78:6509-12. [Crossref] [PubMed]
- Jacobi GH, Wenderoth UK. Gonadotropin-releasing hormone analogues for prostate cancer: untoward side effects of high-dose regimens acquire a therapeutical dimension. Eur Urol 1982;8:129-34. [Crossref] [PubMed]
- Walker KJ, Turkes AO, Turkes A, et al. Treatment of patients with advanced cancer of the prostate using a slow-release (depot) formulation of the LHRH agonist ICI 118630 (Zoladex). J Endocrinol 1984;103:R1-4. [Crossref] [PubMed]
- . Leuprolide Study G. Leuprolide versus diethylstilbestrol for metastatic prostate cancer. N Engl J Med 1984;311:1281-6. [Crossref] [PubMed]
- Crawford ED, Eisenberger MA, McLeod DG, et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med 1989;321:419-24. [Crossref] [PubMed]
- Smith JA Jr, Middleton RG. Implications of volume of nodal metastasis in patients with adenocarcinoma of the prostate. J Urol 1985;133:617-9. [Crossref] [PubMed]
- Pilepich MV, Buzydlowski JW, John MJ, et al. Phase II trial of hormonal cytoreduction with megestrol and diethylstilbestrol in conjunction with radiotherapy for carcinoma of the prostate: outcome results of RTOG 83-07. Int J Radiat Oncol Biol Phys 1995;32:175-80. [Crossref] [PubMed]
- Pilepich MV, John MJ, Krall JM, et al. Phase II Radiation Therapy Oncology Group study of hormonal cytoreduction with flutamide and Zoladex in locally advanced carcinoma of the prostate treated with definitive radiotherapy. Am J Clin Oncol 1990;13:461-4. [Crossref] [PubMed]
- Pilepich MV, Winter K, John MJ, et al. Phase III radiation therapy oncology group (RTOG) trial 86-10 of androgen deprivation adjuvant to definitive radiotherapy in locally advanced carcinoma of the prostate. Int J Radiat Oncol Biol Phys 2001;50:1243-52. [Crossref] [PubMed]
- Pilepich MV, Winter K, Lawton CA, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma--long-term results of phase III RTOG 85-31. Int J Radiat Oncol Biol Phys 2005;61:1285-90. [Crossref] [PubMed]
- Horwitz EM, Bae K, Hanks GE, et al. Ten-year follow-up of radiation therapy oncology group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol 2008;26:2497-504. [Crossref] [PubMed]
- Bolla M, Van Tienhoven G, Warde P, et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol 2010;11:1066-73. [Crossref] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer. Version 2.2018. NCCN Guidelines 2018.
- Jones CU, Hunt D, McGowan DG, et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med 2011;365:107-18. [Crossref] [PubMed]
- Denham JW, Steigler A, Lamb DS, et al. Short-term neoadjuvant androgen deprivation and radiotherapy for locally advanced prostate cancer: 10-year data from the TROG 96.01 randomised trial. Lancet Oncol 2011;12:451-9. [Crossref] [PubMed]
- D'Amico AV, Chen MH, Renshaw AA, et al. Androgen suppression and radiation vs radiation alone for prostate cancer: a randomized trial. JAMA 2008;299:289-95. [PubMed]
- D'Amico AV, Chen MH, Renshaw A, et al. Long-term Follow-up of a Randomized Trial of Radiation With or Without Androgen Deprivation Therapy for Localized Prostate Cancer. JAMA 2015;314:1291-3. [Crossref] [PubMed]
- Narayanan S, Srinivas S, Feldman D. Androgen-glucocorticoid interactions in the era of novel prostate cancer therapy. Nat Rev Urol 2016;13:47-60. [Crossref] [PubMed]
- Mainwaring WI, Williams DC. The effect of castration on the ribonucleic acid metabolism of an experimental prostatic tumour. Biochem J 1966;98:836-40. [Crossref] [PubMed]
- Berges RR, Furuya Y, Remington L, et al. Cell proliferation, DNA repair, and p53 function are not required for programmed death of prostatic glandular cells induced by androgen ablation. Proc Natl Acad Sci U S A 1993;90:8910-4. [Crossref] [PubMed]
- Visakorpi T, Hyytinen E, Koivisto P, et al. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Genet 1995;9:401-6. [Crossref] [PubMed]
- Culig Z, Hobisch A, Cronauer MV, et al. Mutant androgen receptor detected in an advanced-stage prostatic carcinoma is activated by adrenal androgens and progesterone. Mol Endocrinol 1993;7:1541-50. [PubMed]
- Zietman AL, Prince EA, Nakfoor BM, et al. Neoadjuvant androgen suppression with radiation in the management of locally advanced adenocarcinoma of the prostate: experimental and clinical results. Urology 1997;49:74-83. [Crossref] [PubMed]
- Hall EJ, Giaccia AJ. Radiobiology for the radiologist. Eighth edition. Philadelphia: Wolters Kluwer, 2018.
- Bouchaert P, Guerif S, Debiais C, et al. DNA-PKcs expression predicts response to radiotherapy in prostate cancer. Int J Radiat Oncol Biol Phys 2012;84:1179-85. [Crossref] [PubMed]
- Finnie NJ, Gottlieb TM, Blunt T, et al. DNA-dependent protein kinase activity is absent in xrs-6 cells: implications for site-specific recombination and DNA double-strand break repair. Proc Natl Acad Sci U S A 1995;92:320-4. [Crossref] [PubMed]
- Mayeur GL, Kung WJ, Martinez A, et al. Ku is a novel transcriptional recycling coactivator of the androgen receptor in prostate cancer cells. J Biol Chem 2005;280:10827-33. [Crossref] [PubMed]
- Polkinghorn WR, Parker JS, Lee MX, et al. Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov 2013;3:1245-53. [Crossref] [PubMed]
- Evans JR, Zhao SG, Chang SL, et al. Patient-Level DNA Damage and Repair Pathway Profiles and Prognosis After Prostatectomy for High-Risk Prostate Cancer. JAMA Oncol 2016;2:471-80. [Crossref] [PubMed]
- Al-Ubaidi FL, Schultz N, Loseva O, et al. Castration therapy results in decreased Ku70 levels in prostate cancer. Clin Cancer Res 2013;19:1547-56. [Crossref] [PubMed]
- Tarish FL, Schultz N, Tanoglidi A, et al. Castration radiosensitizes prostate cancer tissue by impairing DNA double-strand break repair. Sci Transl Med 2015;7:312re11 [Crossref] [PubMed]
- Goodwin JF, Schiewer MJ, Dean JL, et al. A hormone-DNA repair circuit governs the response to genotoxic insult. Cancer Discov 2013;3:1254-71. [Crossref] [PubMed]


