
Clinical characteristics and prognostic factors of esophageal carcinoma associated with multiple primary carcinomas: a report of 268 cases
Introduction
Multiple primary carcinoma (MPC) is defined as two or more primary carcinomas occurring simultaneously or successively in various parts of the human body. The first case of MPC was reported by Billroth in 1889 (1). In the past few decades, given the extension of human life span, as well as improvements in disease diagnosis and treatment, the incidence of MPC is increasing. It is indisputable that both doctors and patients tend to be more pessimistic and conservative when discussing MPC primarily attributed to insufficient research and proper interpretation. Currently, no standard treatment modality or guidelines has ever been established for patients with MPCs. Relevant information regarding the prognosis of MPCs from various treatment pathways will be useful to guide clinical decision.
Esophageal cancer is 4th common diagnosed cancer in China in 2015 (2). The Chaoshan area of Guangdong Province in southern China is one of the esophageal cancer high-risk area (3). There is limited research in esophageal cancer with multiple primary carcinomas (ECWMPC). Hence, the aim of this study is to retrospectively analyze the clinical characteristics and prognostic factors of ECWMPC patients who underwent surgery treatment pathways, in order to provide a treatment reference.
Methods
Patient selection
This study is approved by the Ethic Board of Shantou University Medical College Cancer Hospital, the Approval ID is 201736. There were 11,066 esophageal carcinoma (EC) patients hospitalized in the Shantou University Medical College Cancer Hospital from 1st January 1996 to 31st December 2012. In this cohort, 330 patients met the diagnostic criteria of ECWMPC. Forty-three of these patients had no complete survival data or follow up information. Among the remaining 287 ECWMPC patients with survival data, 18 patients had three different primary carcinomas, and 1 patient had four. To simplify the analysis, these 19 patients were excluded. The remaining 268 ECWMPC patients who only had two primary cancers were recruited in this study. Additionally, 9,101 single EC patients with complete survival data were analyzed for comparison purposes. All patients received a routine clinical examination and investigations including an endoscope and biopsy, upper gastrointestinal barium X-ray examinations, chest and abdominal enhanced-computed tomography (CT) scans, and blood tests.
Synchronous and metachronous ECWMPC
The diagnosis of MPC was based on the criteria described by Warren and Gates: the tumors must be clearly malignant on histological examination; they must be separated by normal mucosa, and the possibility that the second tumor represents a metastasis must be excluded. MPCs were divided into two categories based on the criteria described by Moertel (4): synchronous for two or more carcinomas diagnosed at the same time or within 6 months, and metachronous for second carcinomas diagnosed more than 6 months from the first carcinoma. In this study, each patient had at least one EC and is stratified as either synchronous ECWMPC (S-ECWMPC) or metachronous ECWMPC (M-ECWMPC).
Treatments
In this study, surgery indicated radical resection of the primary tumor, other than operations with a palliative purpose. Operable esophageal cases were referred to have surgery unless patients or their relatives refused surgical treatment. Thoracic esophagectomy through a right or left thoracotomy with radical node dissection was the standard surgical treatment performed for patients undergoing surgery. Lymphadenectomy involved dissection of lymph node in mediastinum, abdomen and neck regions. Esophagectomy and lymphadenectomy were followed by esophageal reconstruction, mostly using a gastric tube. The surgical resection margins for other primary tumors other than oesophageal carcinoma was based on the most recent NCCN guidelines at the time of treatment.
Only more than 80% of the prescribed dose delivered to the tumor would be accounted as patients received radiotherapy. Chemotherapy refers to at least one cycle of platinum-based chemotherapy. For esophagus cancer radiotherapy, a total dose of 50–66 Gy was delivered using cobalt 60 or linear accelerator in 25–33 fractions. The gross tumor volume (GTV) of the esophageal tumor was contoured according to the results of the barium esophagography, esophagoscopy, or CT scans. The clinical target volume (CTV) included the primary tumor with a 3 cm craniocaudal margin, and the metastatic lymph nodes with a 1 cm margin. Planning target volume (PTV) was defined as CTV plus with a 5 to 10 mm margins for uncertainty. Elective mediastinal lymph nodes received irradiation in most cases. Cervical lymph nodes or supraclavicular lymph nodes would be irradiated for an upper thoracic primary tumor, and celiac lymph nodes would be irradiated for a lower thoracic primary tumor. Three-dimensional CT or X-ray simulation was performed, allowing for two-dimensional anterior-posterior opposed fields and bilateral oblique boosts. After 2008, intensity modulated radiation therapy (IMRT) were increasingly conducted, especially for the upper esophagus. The radiotherapy and chemotherapy for other primary tumors were conducted following the NCCN guidelines.
S-ECWMPC patients who had not received any chemotherapy, radiotherapy or surgery, and M-ECWMPC patients who had no treatment to their second carcinomas were categorized as the “No Treatment” group.
Survival time and follow-up
Survival data were obtained from outpatient review, phone calls, and letters records of patient follow-up records were obtained until 31st May 2017. The survival time of single EC patients was calculated from their cancer diagnosis to death or last visit, whereas the survival time of the ECWMPC patients was calculated from the diagnosis of their second carcinoma.
Statistical analysis
The survival time was calculated from the date of treatment initiation to patient death from any cause or to the last date of survival confirmation. Survival data was updated on 31st May 2017 with a minimum follow-up period of 34 months. Survival curves were estimated using the Kaplan-Meier method and then compared with the log-rank test. Univariable and multivariable Cox proportional hazard models were used to establish the effect of different carcinoma types in particular subgroups. Statistical analysis was performed using the SPSS 19.0 software (SPSS Inc., Chicago, IL, USA). Differences were considered to be significant for P<0.05.
Results
Patient characteristics
For the 9,101 single EC patients, the medium age was 59 [26–92] years, with 6,876 males and 2,225 females, the gender ratio is 3.1:1.
There were 230 males and 38 females among the MPCs with a gender ratio of 6:1. The median ages of first and second carcinoma occurrence were 59 (31 to 85) and 62 (32 to 86) years old, respectively. A univariate analysis of ECWMPC patients indicated that sex, age, smoking history, and family history were not statistically correlated to survival time, with the exception of drinking history (P=0.041) (Table 1).
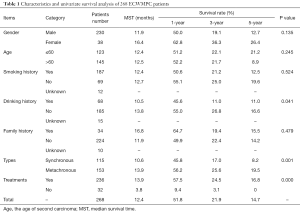
Full table
There was a total of 536 lesions in the ECWMPC patients, among which 287 were esophageal lesions. The locations of esophageal lesions in the cervical, upper, middle and lower thoracic esophagus were 16, 56, 163, and 43 respectively, while another 9 remained unclear. Pathological subtypes were mostly squamous cell carcinoma, with the exception of 3 adenocarcinomas, 2 adenosquamous carcinomas, 1 small-cell carcinoma, and 1 sarcoma. The most common site of carcinoma alongside esophageal cancer was head and neck, followed by the gastric-esophageal junction. Digestive system cancer accounted for 62.3% (167/268). For the subgroup of S-ECWMPC and M-ECWMPC, head and neck remain the popular site of S-ECWMPC but cardia became the top of the M-ECWMPC. More breast and colon rectum disease were found in S-ECWMPC patients (Table 2).
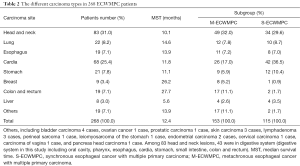
Full table
Treatment detail
In the cohort of 9,101 EC patients with complete survival data, 3,363 patients received operation, 4,625 of them received radiotherapy and/or chemotherapy (RoC), and 843 patients did not receive any treatment.
Among the 115 S-ECWMPC patients, 43 received operations, of which 36 were operated for EC, and 7 for other carcinomas. Fifty-two patients received RoC and 20 patients had no surgical or RoC treatment.
Among the 153 M-ECWMPC cases, 103 patients had surgery, of which 55 were operated for EC, and 48 for other carcinomas. Thirty-eight patients received RoC, and 12 patients had no surgical or RoC treatment for their second carcinomas.
Survival time of EC, total ECWMPC, S-ECWMPC, and M-ECWMPC
The 268 MPC patients had a median survival time (MST) of 12.4 months and a 1, 3 and 5 years overall survival time (OS) were 51.8%, 21.9% and 14.7% respectively. By contrast, the 9,101 single EC patients had a significantly higher OS with an MST of 17.0 months and 1, 3 and 5 years OS of 59.4%, 31.3%, 23.9%, respectively (P<0.001) (Figure 1).
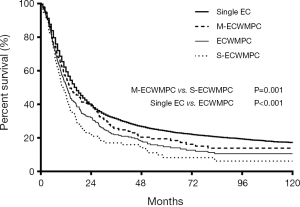
The MST and the OS for 115 S-ECWMPC patients with a 1, 3, and 5 years survival time were 10.6 months and 45.8%, 17.0%, 8.2%, respectively. By contrast, the MST and the OS for 153 M-ECWMPC patients were 13.9 months and 56.2%, 25.6%, 19.5%, respectively. This showed significant difference with P=0.001 (Figure 1). Therefore, it was shown that patients who had received treatment had better survival than those who did not receive treatment.
Survival comparison in S-ECWMPC with different treatments
In 115 cases of S-ECWMPC, 43 patients received surgery (at least one primary carcinoma resected), and had a better survival compared to those 52 patients who only received RoC (P=0.031) (Figure 2). These 52 patients who had received RoC had a better survival than the remaining 20 patients who received no treatment (P=0.002) (Figure 2). These remaining patients had an MST and an OS at 1, 3, and 5 years was of 4.1 months, 10.0%, 5.0%, and 0%, respectively.

Compared to the 3,633 EC patients who had surgery, those 43 patients who were operated on had a worse survival (P=0.003) (Figure 2), with an MST and OS at 1, 3, and 5 years of 16.4 months, 64.8%, 33.3%, and 18.6% respectively. This is similar to the survival time of the 4,625 EC patients who had received RoC (P=0.253) (Figure 2). Finally, the 52 S-ECWMPC patients who had received RoC had the worst survival time, with an MST and an OS at 1, 3, and 5 years of 9.8 months, 44.2%, 8.7%, and 3.2% respectively.
Survival comparison in M-ECWMPC with different treatment and ITD
In the 153 cases of M-ECWMPC, the 103 patients who had received surgery (at least one primary carcinoma resected) had a better survival time, with an MST and OS at 1, 3, and 5 years of 22.5 months, 66.0%, 31.8%, and 25.4%, respectively, than the 38 patients who only received RoC, with an MST and OS at 1, 3, and 5 years of 10.6 months, 44.7%, 16.9%, and 8.5% respectively (P=0.026) (Figure 3). However, this was significantly worse than those 3,633 operated EC patients whose MST and OS survival time at 1, 3, and 5 years was 31 months, 76.1%, 47.1%, and 37.0%, respectively (P=0.004) (Figure 3). Finally, the 38 patients who had received RoC had better survival time than the 12 patients who had no treatment (P<0.001) (Figure 3).
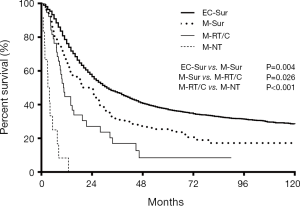
Among the 103 M-ECWMPC patients who had received surgery, 55 of them had an esophagus resection, with an MST and OS at 1, 3, and 5 years of 30 months, 80.0%, 42.3%, and 37.7%, respectively. This was a similar survival time to those 3,633 operated EC patients (P=0.905) (Figure 4). Finally, the remaining 48 patients who had non-esophageal surgery had an MST and OS at 1, 3, and 5 years of 11.7 months, 50.0%, 19.7%, and 10.6%), respectively. This was a similar survival time to those 38 patients who had only received RoC (P=0.983) (Figure 4).
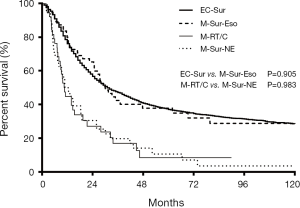
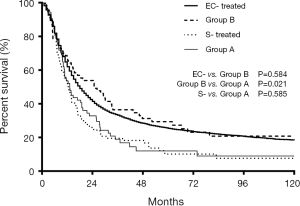
The median ITD of the 153 M-ECWMPC patients in this study was 2.9 (0.5–20.6) years. Using a cut-off of 3 years, 141 treated patients were stratified as group A (71 cases) for those within a 3-year ITD, and group B (70 cases) for an ITD of more than 3 years. Survival analysis showed a significant difference between the 2 groups, with group B having a better prognosis (P=0.021) (Figure 5). Group B MST and OS survival time at 1, 3, and 5 years was 24.8 months, 67.1%, 36.4%, and 29.4%, respectively. This was similar survival time to the 8,258 treated single EC patients (P=0.584) (Figure 5) that had an MST and OS survival time at 1, 3, and 5 years of 18.0 months, 62.0%, 33.2%, and 25.4%, respectively. Interestingly, there was no statistical difference (P=0.585) between the survival curves of group A, with an MST and OS at 1, 3, and 5 years of 13.2 months, 53.5%, 18.9%, and 12.0%, respectively, and the 95 treated S-ECWMPC patients, with an MST and OS at 1, 3, and 5 years of 12.7 months, 53.4%, 19.5%, and 10.1%, respectively (Figure 5).
Discussion
The average ages of single EC patients and MPCs patients in this study are 59, which fit in with the demographic of Chinese patients with esophageal cancers (5). There is also a higher proportion of male patients with MPCs compared to patients with single esophagus carcinomas as illustrated by the higher gender ratio of 6:1 vs. 3.1:1. A higher prevalence of male smokers and alcoholics in China can result in not an increased risk to developing oesophageal cancers (6) but also an increased risk to develop a second tumor. This study showed that only alcohol drinking history is a significant prognostic factor among all the life styles habits (P=0.041), it is known that alcohol drinking was associated with overall mortality, especially among smokers and esophageal cancer patients (6,7).
Among the 268 MPC patients, only 7 of them have a non-squamous cell carcinoma in the esophagus. It is reported that around 90% of Chinese will have esophageal squamous cell carcinoma (8). Thought the ratio of the esophageal squamous cell is higher but it is not conclusive to assume it is related to a higher tends to develop secondly tumor in a background that squamous cell is way more common than others.
Preexisting knowledge in relation to cancer development struggles to explain the occurrence of synchronous and metachronous carcinomas in both the head and neck region and in the esophagus (9). While recent research shows that the overexpression of p53 may be a biological marker in mediating MPCs (10,11), the real pathogenesis of MPCs is still under investigation. Multiple risk factors, including race, living environment, lifestyle (for example, alcohol, smoking, and pickles), genetic susceptibility, and the immune status are believed to play a significant role in the development of the disease. In this study, show the most common site alongside esophageal cancer was head and neck, the reason could be quite a high rate of smoking history (69.8% 187/268), and Guangdong Province also have a high incidence of nasopharyngeal carcinoma, that could be another reason. Furthermore, head and neck cancers have a better survival, might cause the patients have the chance to develop the second malignancy. Interestingly, in the subgroup analyze, M-ECWMPC group have more gastric-esophageal junction (36.5% vs. 17.0%) and stomach (10.4% vs. 5.9%) second malignance then the S-ECWMPC group. Head and neck remains almost the same (29.6 vs. 32.0%), but less breast (0.9% vs. 5.2%) and colon-rectum (1.7% vs. 11.1%) in M-ECWMPC. The reason could be more likely the secondly malignance share the same risk factors which cause the esophagus in the M-ECWMPC patients. Also the previous treatment, especially radiotherapy may be a significant factor too (12), the upper digestive system might got radiated during the previous treatment. Breast and colon-rectum malignance have relatively independence cause, it is more likely accidental discoveries rather than exposed to the same risk factor.
Between 1975 and 2001, a large study with about nine million cancer patients in the USA was statistically analyzed, revealing a 7.9% (13) incidence rate of MPC. The incidence rate of ECWMPCs among ECs in other reports is between 9.5–24.1% (14,15), compared to 3.0% found in this study. A probable reason for this may be a misdiagnosis. In the follow-up setting, patients found to have a second tumor could be easily regarded as a treatment failure or metastasis. Many patients and their family may also lose confidence or decline to receive additional medical care if another cancer appears, especially in cases involving short time intervals between the occurrences of two cancers.
MPCs were usually accompanied with a poor prognosis (16), which was also true in this study. For example, the MST of 268 patients was 12.4 months, compared to 16.1 months of single EC patients, with P<0.001. As the gender ratio was 6 to 1, males were prone to MPCs. A possible reason for this might be because males had more likely to smoke, drink alcohol and be associated with other risks. This study also found median ages of the first and second carcinomas as 59 and 62, which is consistent with other reports stating MPCs is more prevalent in elderly patients (17,18). EC accompanied with hepatocellular carcinoma was also found to have a worse prognosis, with an MST of only 5.6 months, whereas EC alongside breast or colorectal cancer had a better prognosis, with an MST of 26.2 and 27.7 months, respectively. However, survival analysis did not show significant differences among ECs alongside other cancers (P=0.368) (Table 2)
Given the particularity of MPCs, there was no calculation of a unified standard survival time. Obviously, MPCs could only occur in patients who had been diagnosed with a carcinoma already and had survived until a second cancer developed. The longer the survival, the higher incidences of MPCs the cancer patients would have. The 1-, 3-, and 5-year survival time of MPCs was usually very close to those single carcinomas if survival time was calculated from the first carcinoma and this seems inconsistent with the facts. Therefore, the survival time of MPCs in this study was calculated from the last carcinoma and was defined as ‘multiple primary carcinoma OS’.
The MST of M-ECWMPC and S-ECWMPC were 13.9 and 10.6 months, respectively, with P=0.001. This result is supported by other reports in which metachronous multiple cancers had a better prognosis (19). It is not difficult to understand that synchronous multiple cancer patients suffered from two or more carcinomas in a short period of time, which may have produced larger volume tumors because a synergistic effect may exist in these cancers. The consequences of this condition would weaken the patients and increase the difficulties and effects of treatments. For example, the surgical resection rate (at least one lesion removed) in S-ECWMPC patients in this study was only 35.7%, which is significantly lower when compared to the rate of 70.8% in M-ECWMPC patients.
In 115 cases of S-ECWMPC, the 43 patients who had received surgery (at least one primary carcinoma surgery resection) had a better survival compared to those 52 patients who only received RoC (P=0.006). However, both of these groups had poorer survival than the single EC patients who were operated on. It is not hard to understand that S-ECWMPC patients might have had a poorer general condition because more than one type of carcinoma may have developed at the same time. Therefore, analyzing patient survival times become more complicated and more damage may be caused to the patient. However, the patients who had surgery still show a better survival time; thus, given these results, surgery may be a highly recommended treatment.
In the 153 cases of M-ECWMPC, the 103 patients who had received at least one primary carcinoma surgery resection survived longer than the 38 patients who only received RoC, though this is shorter than for the 3,633 EC patients who were operated upon. To further investigate the effect of surgery, the 103 patients were divided into two groups based on whether they had received esophageal surgery. The 55 patients who had received esophageal surgery had a similar survival time as those 3,633 EC patients who were operated on (P=0.905). In addition, the remaining 48 patients who had received non-esophageal surgery had a similar survival time as those 38 patients who only received RoC. There are quite a few reasons that may explain this result. Firstly, compared to the second primary carcinoma, which is mostly a head and neck carcinoma, ECs have a poorer prognosis. This might mean that survival times are directly dependent on a treatment strategy of the esophageal. Secondly, operable patients always have an earlier stage of cancer and thus are in better general health. Thirdly, patient treatments can date back to 1996, when radiotherapy was the main treatment for the inoperable esophageal patients and concurrent chemoradiotherapy was rarely applied. JCOG 9906 research reported that stage II–III esophageal squamous cell carcinoma treated by concurrent chemoradiotherapy were found to have 3- and 5-year survival rates at 44.7% and 36.8% (20), respectively. Therefore, this study proves that concurrent chemoradiotherapy might have the same survival as surgery in esophageal patients. However, further research is needed in order to evaluate the extent to which these new treatment techniques can benefit inoperable patients.
In relation to the M-ECWMPC setting, those treated patients with a more than 3 years ITD had a much better survival than those with an ITD of less than 3 years (P=0.021). Interestingly, this was similar to that of the treated single EC patients (P=0.584), whereas treated M-ECWMPC patients with a less than 3 years ITD had a poorer survival time, which was similar to S-ECWMPC patients (P=0.585).
A plausible reason for this might be a long ITD allows patients to recover more effectively, thus, these patients might be able to tolerate later aggressive treatment. From this point of view, more active modalities should be considered for M-ECWMPC patients with a longer ITD, which may turn out to produce better results. It was not hard to decide that treatment strategies were different for the two categories. For M-ECWMPC patients with a more than 3 years ITD, the focus was on a treatment strategy for their last carcinoma. More specifically, the patient tolerance for retreatment as the comprehensive treatment of the first carcinoma often lead to the decline of immune function. However, for M-ECWMPC patients with a less than 3 years ITD, the first carcinoma affected OS and the side effects of the first treatments could not be ignored; thus, treatment options became more individualized and complex. Given that the occurrence of the second carcinoma could not be predicted, follow-up and regular re-examination should not be excluded for cancer patients after treatments.
In this study, patients who had received at least one type of treatment for their last cancer had a better survival (P<0.001, Table 1) in comparison to the 32 patients who either refused treatment at the very beginning in the S-ECWMPC setting, or did not received treatment for the last diagnosed cancer in the M-ECWMPC group. Therefore, this result strongly demonstrated that treatments would benefit patient survival time.
In other reports, the incidence rate of ECWMPCs among ECs was 9.5–24.1%, and a routine re-examination is necessary for those treated EC patients. It not only can evaluate the treatment outcome of the original tumor, but also can discover the secondary tumor as early as possible. In other words, early detection means more chance a patient can have surgery, which can lead to a better survival time. National Comprehensive Cancer Network (NCCN) guidelines recommend performing the following examinations in the initial workup of esophageal cancer: upper gastrointestinal endoscopy and biopsy, chest and abdominal CT, PET evaluation, EUS, and bronchoscopy (21,22) (if the tumor is at or above the carina).
There were some limitations of this study: firstly, clinical and survival data were collected from 1st January 1996 to 31st December 2012, the change of medical treatment and techniques, and increasing economic and social development in these 17 years might have affected this data. Secondly, the survival curves of surgery for the RoC group may have been influenced by tumor stage or other molecular target therapy, and thus could not be estimated in this study. Finally, as this is a retrospective study, the surgery treatment recommendations need to be confirmed by further prospective randomized clinical trials.
Conclusions
ECWMPC is not rare among ECs. The predilection sites were found to be in the head and neck, followed by the gastric-esophageal junction. Alcohol drinking history is associated with overall mortality. There is more gastric-esophageal junction and stomach second malignancy in M-ECWMPC group, head and neck remain almost the same but breast and colon-rectum are less compare to the S-ECWMPC. Treatments have been shown to strongly benefit survival times. In addition, the prognosis of M-ECWMPC patients was found to be better than that of S-ECWMPC patients. M-ECWMPC patients with a greater than 3 years ITD had a better prognosis than those patients with a less than 3 years ITD. Moreover, patients who had surgery have been shown to have a better survival time than those patients who had RoC for S-ECWMPC and M-ECWMPC. In M-ECWMPC patients, esophageal surgery has been shown to acquire a better survival benefit than other treatments. Therefore, given these results, this study highly recommends esophageal surgery as a method for treating ECWMPC.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.07.11). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study is approved by the Ethic Board of Shantou University Medical College Cancer Hospital, the Approval ID is 201736. The written informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Warren S, Gates O. Multiple primary malignant tumors: survey of the literature and a statistical study. Am J Cancer 1932;16:1358-414.
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Su M, Liu M, Tian DP, et al. Temporal trends of esophageal cancer during 1995-2004 in Nanao Island, an extremely high-risk area in China. Eur J Epidemiol 2007;22:43-8. [Crossref] [PubMed]
- Moertel CG, Dockerty MB, Baggenstoss AH. Multiple primary malignant neoplasms. II. Tumors of different tissues or organs. Cancer 1961;14:231-7. [Crossref] [PubMed]
- Jiang YX, Zhang DW, Chen Y, et al. The characteristics of oesophageal squamous cell carcinoma: an analysis of 1317 cases in southeastern China. Contemp Oncol (Pozn) 2015;19:137-41. [Crossref] [PubMed]
- Yang L, Zhou M, Sherliker P, et al. Alcohol drinking and overall and cause-specific mortality in China: nationally representative prospective study of 220,000 men with 15 years of follow-up. Int J Epidemiol 2012;41:1101-13. [Crossref] [PubMed]
- Wu M, Zhao JK, Zhang ZF, et al. Smoking and alcohol drinking increased the risk of esophageal cancer among Chinese men but not women in a high-risk population. Cancer Causes Control 2011;22:649-57. [Crossref] [PubMed]
- Lin Y, Totsuka Y, He Y, et al. Epidemiology of Esophageal Cancer in Japan and China. J Epidemiol 2013;23:233-42. [Crossref] [PubMed]
- Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer 1953;6:963-8. [Crossref] [PubMed]
- Homann N, Nees M, Conradt C, et al. Overexpression of p53 in tumor-distant epithelia of head and neck cancer patients is associated with an increased incidence of second primary carcinoma. Clin Cancer Res 2001;7:290-6. [PubMed]
- Martin-Ezquerra G, Salgado R, Toll A, et al. Multiple genetic copy number alterations in oral squamous cell carcinoma: study of MYC, TP53, CCDN1, EGFR and ERBB2 status in primary and metastatic tumours. Br J Dermatol 2010;163:1028-35. [Crossref] [PubMed]
- Watanabe S. Epidemiology of multiple primary cancer. Gan To Kagaku Ryoho 1990;17:967-73. [PubMed]
- Mariotto AB, Rowland JH, Ries LA, et al. Multiple cancer prevalence: a growing challenge in long-term survivorship. Cancer Epidemiol Biomarkers Prev 2007;16:566-71. [Crossref] [PubMed]
- Natsugoe S, Matsumoto M, Okumura H, et al. Multiple primary carcinomas with esophageal squamous cell cancer: clinicopathologic outcome. World J Surg 2005;29:46-9. [Crossref] [PubMed]
- Lee GD, Kim YH, Kim JB, et al. Esophageal Cancer Associated with Multiple Primary Cancers: Surgical Approaches and Long-term Survival. Ann Surg Oncol 2013;20:4260-6. [Crossref] [PubMed]
- Morita M, Yoshida R, Ikeda K, et al. Advances in esophageal cancer surgery in Japan: an analysis of 1000 consecutive patients treated at a single institute. Surgery 2008;143:499-508. [Crossref] [PubMed]
- Arai T, Sawabe M, Takubo K, et al. Multiple colorectal cancers in the elderly: a retrospective study of both surgical and autopsy cases. J Gastroenterol 2001;36:748-52. [Crossref] [PubMed]
- Luciani A, Balducci L. Multiple primary malignancies. Semin Oncol 2004;31:264-73. [Crossref] [PubMed]
- Kim C, Chon H, Kang B, et al. Prediction of metachronous multiple primary cancers following the curative resection of gastric cancer. BMC Cancer 2013;13:394. [Crossref] [PubMed]
- Kato K, Muro K, Minashi K, et al. Phase II study of chemoradiotherapy with 5-fluorouracil and cisplatin for Stage II-III esophageal squamous cell carcinoma: JCOG trial (JCOG 9906). Int J Radiat Oncol Biol Phys 2011;81:684-90. [Crossref] [PubMed]
- Lou F, Sima CS, Adusumilli PS, et al. Esophageal cancer recurrence patterns and implications for surveillance. J Thorac Oncol 2013;8:1558-62. [Crossref] [PubMed]
- Oppedijk V, van der Gaast A, van Lanschot JJ, et al. Patterns of recurrence after surgery alone versus preoperative chemoradiotherapy and surgery in the CROSS trials. J Clin Oncol 2014;32:385-91. [Crossref] [PubMed]


