
Social factors, treatment, and survival in patients with advanced-stage non-small cell lung cancer
Introduction
Lung cancer is the leading cause of cancer death in North America. It is estimated that 226,160 men and women (116,470 men and 109,690 women) will be diagnosed with and 160,340 men and women will die of lung cancer in 2012 (1). And at least 40% of patients already have advanced, incurable, metastatic (stage IV) disease at the time of diagnosis (2). Non-small cell lung cancer (NSCLC) is the dominant histology responsible 85% of all lung cancers in the United States according to recent Surveillance Epidemiology and End Results (SEER) data (1). The overall 5-year relative survival for 2002-2008 from 18 SEER geographic areas is just 15.9% and less than 4% in the subset presenting with distant metastases (1).
The introduction of platinum-doublet chemotherapy in the 1990’s for advanced lung cancer saw modest survival gains, increasing median survival by about 11/2 months, and survival at 1 year from 5% to 15% (3). Since then the development of tyrosine kinase inhibitors (erlotinib and gefitinib) in the setting of epidermal growth factor receptor (EGFR) mutations have been found to be effective as monotherapies in the second-line setting and in EGFR mutation-positive tumors in the first-line setting (4-6). The discovery of angiogenesis as a promising drug target led to the development of bevacizumab, a monoclonal antibody against vascular endothelial growth factor (VEGF) that has shown benefit in combination with platinum-based doublet chemotherapy in patients with nonsquamous NSCLC (7). Finally, improvements in maintenance chemotherapy and the early introduction of palliative care have improved survival to approximately 11.6 months (8,9).
Chemotherapy has a well-defined role in the treatment of patients with advanced NSCLC. Compared with best supportive care, chemotherapy prolongs overall survival without significantly impairing overall quality of life (10). Nevertheless, population-based studies from the 1990s have shown that only 22% to 31% of patients with advanced NSCLC ever receive chemotherapy (11-14). Potential reasons for this seemingly low treatment rate include advanced patient age, poor performance status, and comorbidities; referring and treating physician practice patterns; and patient preference (11). However, these studies were taken from a time frame that did not reflect contemporary treatment patterns and were limited to Medicare billing codes through SEER data that excluded patients under 65 years of age. Most recently Rasco et al. performed a retrospective analysis of 718 patients from 2000 to 2007 using tumor registries on all patient ages at the University of Southwestern Texas Medical Center and found that 49% of patients with advanced NSCLC received chemotherapy (15).
Given the considerable changes to advanced NSCLC care in recent years including the introduction of innovated diagnostic modalities and new targeted and less toxic chemotherapeutic regimens, we performed a retrospective data analysis between 2009 and 2010 from two community-based academic hospitals via the Scripps Cancer Registry to define factors that influence the delivery of treatment and assess their impact on overall survival. We hypothesized that this cohort would have substantially higher rates of chemotherapy administration than previous reported studies. In addition we hypothesize that vast differences in socioeconomic status and referral patterns between the two hospital populations could account for differences in treatment and overall survival.
Methods
Study setting
This study was approved by the Scripps Institutional Review Board. The study sample was drawn from the Scripps Cancer Registry and electronic medical record data from Scripps Mercy Hospital (SMH) and Scripps Green Hospital (SGH). SMH consists of a 517-bed acute-care hospital and outpatient clinics serving San Diego’s primarily indigent population. SMH oncology clinic serves as the primary referral clinic for the majority of inpatient oncology consultations. SGH (173 beds) primarily serves La Jolla, California which does not have an emergency department or the infrastructure to serve the indigent population with most oncology consultations in the outpatient setting. Both hospitals are located in San Diego County in southern California.
Patient data extraction
Patients diagnosed with stage IV NSCLC at SMH and SGH between January 1, 2009 and December 31, 2010 were identified though the Scripps Cancer Registry. The 2009-2010 timeline was selected because of two major advancements in advanced NSCLC treatment: namely the IRESSA Pan-Asia Study (IPASS) advocating routine EGFR screening for non-squamous cell tumors and the use of less-toxic TKIs as first-line therapy in the setting of EGFR-mutation positive tumors, and the introduction of early palliative care which demonstrates improved quality of life and overall survival (11,16). Patient data were obtained from the Scripps Cancer Registry and through review of pathology records, hospital admission and discharge records, outpatient clinic progress notes, and the Social Security Index to confirm dates of death. The Scripps Cancer Registry is overseen by registrars credentialed by The National Cancer Registrars Association to ensure the collection of timely, accurate, and complete data. The registry collects data on all patients diagnosed and treated at a Scripps facility with a malignant diagnosis. The information is reported to the California Cancer Registry and the Commission on Cancer National Cancer Data Base. The registry also does annual lifetime follow-up on all patients in their database.
Definition of variables
Data were abstracted from the Scripps Cancer Registry with the following variables: TNM stage, age, gender, place of diagnosis, insurance, histology, ECOG performance status at diagnosis, date of diagnosis, date of death, treatment given or not given, time to treatment, referral to hospice, EGFR testing, EGFR mutation status and survival time. TNM stage IV was based on the 7th Edition American Joint Commission on Cancer Staging Manual published in 2010 and the previous 6th Edition for 2009 data by incorporating stage IIIB currently classified as stage IV disease by contemporary guidelines (17-19). ECOG performance status is a 0-5 scale used to assess how the disease affects the daily living abilities of the patient (20). Treatment defines whether the patient received palliative chemotherapy and/or radiation following diagnosis. Survival time is the length of time from diagnosis via tissue pathology until death or August 1, 2012, the date of data truncation for this manuscript.
Statistical data analysis
Baseline characteristics and outcomes of patients seen at the two hospitals were compared using Chi square tests for categorical variables and non-parametric tests (Wilcoxon rank sum and Kruskall-Wallis) for continuous variables. All statistical analyses were performed using SAS version 9.2 (SAS, Cary, North Carolina).
Results
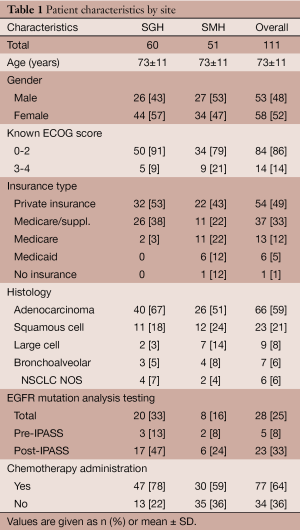
In all, 111 patients were included in the study. Of those patients, the median age was 73 years (range, 45-95 years) and 58 patients (52%) were women (Table 1). Adenocarcinoma (59%) was the most common type of cancer, followed by squamous cell (21%), large cell (8%), and bronchoalveolar (6%). Sixty patients (54%) received care at SGH and 51 patients (46%) received care at SMH. There were 54 patients (49%) with private health insurance, 37 patients (33%) with Medicare and private supplemental insurance, 13 patients (12%) with Medicare, 6 patients with Medicaid (5%), and 1 patient (1%) with no insurance. The ECOG status was known of 98 patients (88%), of whom 84 patients (86%) had an ECOG score between 0-2, compared to 14 patients (14%) with ECOG 3 or greater.
Full table
Chemotherapy analysis
Overall, 77 patients (64%) received chemotherapy. In univariate analysis, place of diagnosis was associated with receipt of chemotherapy as 78% of SGH patients received chemotherapy, compared with 59% of SMH patients (P=0.03). The median time to treatment for SGH patients was 0.5 months compared to 0.9 months to SMH patients, however this did not reach statistical significance (P=0.38). The median ECOG status for SGH and SMH patients were 1.00 and 2.00, respectively (P=0.05). Overall, 81% of patients with ECOG scores of 0-2 received chemotherapy with no significant difference between hospital sites (86% at SGH vs. 74% at SMH, P=0.15). In comparison, of the 14 patients with ECOG scores of 3-4, none received chemotherapy (P<0.001). Of those tested for the EGFR mutation, 96% received chemotherapy compared to 60% of those not tested (P<0.001). There was also a nonsignificant trend towards chemotherapy administration to women (78%), compared to men (60%).
Survival analysis
Median survival for all patients was 6.3 months. There was no significant difference in median survival between hospitals (7.2 months for SGH versus 5.1 months for SMH, P=0.51). Patients that received chemotherapy had a median survival of 9.8 months, compared to 1.9 months for those that did not receive chemotherapy (P<0.001). At SGH, chemotherapy patients had a median survival of 9.6 versus 1.0 months without chemotherapy (P<0.001). At SMH, patients who received chemotherapy had a median survival of 11.7 months versus a median survival of 1.9 months without chemotherapy (P<0.001). Overall, median survival for ECOG performance status 0-2 patients was 8.4 months, compared with 0.5 months for patients whose ECOG status was 3-4 (P<0.001).
Discussion
To date, this may be the only academic community-based study reviewing rates of chemotherapy administration and survival for advanced NSCLC reflecting contemporary disease management. In this study, 64% of overall patients with advanced NSCLC received chemotherapy, an improvement from earlier studies from the mid-2000’s in which approximately half of patients received chemotherapy (16). These data likely reflect major advances in NSCLC treatment and survival since 2009 along with the advent of further diagnostic modalities. Those patients treated at SGH, mostly privately insured patients with favorable ECOG scores, were more likely to receive chemotherapy than at SMH which serves a more indigent population (78% vs. 59%, P=0.03). In addition, median survival was approximately five times longer amongst patients who received chemotherapy compared to those who did not.
Relative to previously reported figures, our study suggest that chemotherapy utilization is increasing over time. From 1985 to 1989 Smith et al. reported that 4.2% of advanced NSCLC patients received chemotherapy (21). That rate increased between 1989 and 1991 to 18.8%, and most recently 49% of patients between 2000 and 2007 received chemotherapy (12,22). That 64% of patients with advanced NSCLC received chemotherapy for their lung cancer between 2009 and 2010 indicates that chemotherapy use is increasing relative to the rates reported in older studies. This corresponds temporally to several major randomized chemotherapeutic trials including the introduction of modern generation doublet chemotherapy, tyrosine kinase inhibitors, and monoclonal antibodies directed against VEGF (23,24). EGFR mutation analysis for non-squamous cell cancer allows for front-line and less-toxic targeted therapy, in the form of tyrosine kinase inhibitors such as erlotinib. The introduction of early palliative care offers an approach to advanced NSCLC with known survival benefits; a separate analysis of the impact of palliative care on this data set is currently underway (9). Given that 14% of patients in this study had late-stage disease (PS of ECOG 3-4 at time of diagnosis)—for whom the benefits of chemotherapy remain controversial—it remains to be seen whether further advances in the field will lead to increased use of targeted and less toxic therapy. Accordingly, crizotinib, an oral anaplastic lymphoma kinase (ALK) inhibitor, and erlotinib have shown benefits in ALK positive and EGFR mutated tumors, respectively, even in patients with poor performance status who would otherwise be poor candidates for chemotherapy (25-27). This could be applicable to up to 25-30% of patients with adenocarcinoma as mutually exclusive EGFR and ALK mutations have been found in approximately 20% and 4-7% of those tumors, respectively (28-31).
A significant difference in chemotherapy administration between SGH and SMH reflect a multitude of confounding factors in this community-based cohort. In our study 97% of SGH patients had some form of private insurance, compared to 63% of SMH patients (Table 1). The diagnosis-to-treatment interval—which encompasses radiographic interpretation, a confirmatory imaging study (in some cases), scheduling and performing a biopsy, and pathology interpretation—has been shown to have a significant correlation with hospital type and insurance type in the NSCLC setting (32). A 2009 study by Yorio et al. analyzed differences NSCLC between public and private hospital settings and found a pronounced time-to-treatment range according to insurance status: 50 days for patients with private insurance compared to 140 days for patients with Medicaid (32). It is likely that administrative requirements, coordination of care, transportation, social work and insurance authorizations all represent barriers to treatment amongst the underinsured while hospitalized. However, in spite of this we found no association between the diagnosis-to-treatment interval and overall survival, an observation consistent with earlier studies (32-35).
In accordance with current National Comprehensive Cancer Network (NCCN) guidelines, it has been demonstrated that patient’s with improved performance status are more likely to be offered chemotherapy for advanced NSCLC (36). The median ECOG status for SGH and SMH patients were 1.00 and 2.00, respectively (P=0.05) at the time of diagnosis. Accordingly, SGH patients received treatment more frequently than SMH patients (P=0.03). Current recommendations support chemotherapy administration to selected ECOG performance status 0-2 patients as there has been a demonstrated survival benefit; however the same correlation has not been demonstrated in ECOG 3-4 patients (23). When one considers that underinsured patients are less likely to have access to medical care or participate in routine cancer screening, it is not surprising that underinsured SMH patients in our cohort had a poorer ECOG status than their privately insured SGH counterparts (37). In fact, it was recently shown that underinsured patients with lung cancer have a substantially increased risk of presenting with advanced-stage cancers at diagnosis (38). To our knowledge, the correlation between EGFR mutation analysis and chemotherapy administration has not been shown. Of those tested for the EGFR mutation, 96% received chemotherapy compared to 60% of those not tested (P<0.001). This likely reflects trends in contemporary treatment towards less-toxic TKIs which have been shown to increase progression-free survival for selected patients.
This study has several limitations. First, the SEER database and Scripps Cancer Registry exclude information on which therapeutic agents were prescribed, as well as their order of use. Accordingly, it would be interesting to know whether patients with EGFR-mutation positive tumors received erlotinib as front-line therapy, and whether non-traditional chemotherapy candidates, such as those with an ECOG PS of 3, were screened for the EGFR mutation. As crizotinib was recently approved by the Food and Drug Administration for patients with advanced NSCLC and the ALK gene rearrangement, another area of future study in a more current data set could be to determine the frequency of crizotinib use in the setting of ALK-positive tumors (39). Second, the study cohort was drawn from two community-based hospitals in San Diego, California, thus geographically limiting the generalizability of our data. Nevertheless, wide variations in demographics and funding sources between the two institutions show that this is a socioeconomically diverse population. Third, patients without definitive pathology were excluded from the study. This includes patients with late-stage and metastatic disease of unconfirmed origin who never had a confirmatory biopsy. In addition, this retrospective analysis relies on data within SEER and the Scripps Cancer Registry which excludes incomplete records. From a demographic perspective, we were unable to differentiate race within the cohort. Finally, our data assembly excluded patients with recurrent lung cancer and patients who presented to us after progression of earlier stage lung cancer.
In conclusion, in this contemporary, socioeconomically diverse cohort of patients with advanced NSCLC, 64% of patients received chemotherapy. This correlates with recent improvements in survival, likely owing to advancement in modern diagnostic and treatment modalities. Chemotherapy administration is associated with performance status, place of diagnosis and insurance status at the time of diagnosis. As chemotherapy administration conferred a significant survival benefit amongst both patient cohorts (1.0 months compared to 9.6 months at SGH; 1.9 months compared to 11.7 months at SMH, P<0.001), it is hoped that further developments in this field will lead to an increase in patients in community settings who would benefit from chemotherapy or less-toxic targeted agents.
Acknowledgments
The authors thank Jill Waalen, MD from the Scripps Translational Science Institute and Anika Garcia-Smith from the Scripps Cancer Registry for their assistance and contributions.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2013.07.01). MX serves as an unpaid editorial board member of Translational Cancer Research. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki. This study was approved by the Scripps Institutional Review Board. Informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Howlader N, Noone A, Krapcho M, et al. SEER cancer statistics review. Available online: http://seer.cancer.gov/csr/1975_2008/
- Ihde DC. Chemotherapy of lung cancer. N Engl J Med 1992;327:1434-41. [PubMed]
- Ettinger DS, Cox JD, Ginsberg RJ, et al. NCCN non-small-cell lung cancer practice guidelines. The national comprehensive cancer network. Oncology (Williston Park) 1996;10:81-111. [PubMed]
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353:123-32. [PubMed]
- Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) J Clin Oncol 2003;21:2237-46. [corrected]. [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [PubMed]
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542-50. [PubMed]
- Bunn PA Jr, Kelly K. New chemotherapeutic agents prolong survival and improve quality of life in non-small cell lung cancer: a review of the literature and future directions. Clin Cancer Res 1998;4:1087-100. [PubMed]
- Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet 2009;374:1432-40. [PubMed]
- Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010;363:733-42. [PubMed]
- Earle CC, Venditti LN, Neumann PJ, et al. Who gets chemotherapy for metastatic lung cancer? Chest 2000;117:1239-46. [PubMed]
- Tannock IF, Boyer M. When is a cancer treatment worthwhile? N Engl J Med 1990;323:989-90. [PubMed]
- . Clinical practice guidelines for the treatment of unresectable non-small-cell lung cancer. Adopted on May 16, 1997 by the American society of clinical oncology. J Clin Oncol 1997;15:2996-3018. [PubMed]
- Lopez PG, Stewart DJ, Newman TE, et al. Chemotherapy in stage IV (metastatic) non-small-cell lung cancer. Provincial lung disease site group. Cancer Prev Control 1997;1:18-27. [PubMed]
- Rasco DW, Yan J, Xie Y, et al. Looking beyond surveillance, epidemiology, and end results: patterns of chemotherapy administration for advanced non-small cell lung cancer in a contemporary, diverse population. J Thorac Oncol 2010;5:1529-35. [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [PubMed]
- Edge S, Byrd D, Compton C, et al. eds. AJCC cancer staging manual (7th Ed). New York: Springer, 2010.
- Greene F, Page D, Fleming I, et al. eds. AJCC cancer staging manual (6th Ed). New York: Springer-Verlag, 2002:435.
- Sobin LH, Wittekind Ch. eds. UICC TNM classification of malignant tumors 6th edition. New York: Wiley-Liss, 2002:272.
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649-55. [PubMed]
- Smith TJ, Penberthy L, Desch CE, et al. Differences in initial treatment patterns and outcomes of lung cancer in the elderly. Lung Cancer 1995;13:235-52. [PubMed]
- Hillner BE, McDonald MK, Desch CE, et al. A comparison of patterns of care of nonsmall cell lung carcinoma patients in a younger and medigap commercially insured cohort. Cancer 1998;83:1930-7. [PubMed]
- Azzoli CG, Baker S Jr, Temin S, et al. American society of clinical oncology clinical practice guideline update on chemotherapy for stage IV non-small-cell lung cancer. J Clin Oncol 2009;27:6251-66. [PubMed]
- Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol 2009;27:1227-34. [PubMed]
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693-703. [PubMed]
- Shaw AT, Solomon B. Targeting anaplastic lymphoma kinase in lung cancer. Clin Cancer Res 2011;17:2081-6. [PubMed]
- Goss G, Ferry D, Wierzbicki R, et al. Randomized phase II study of gefitinib compared with placebo in chemotherapy-naive patients with advanced non-small-cell lung cancer and poor performance status. J Clin Oncol 2009;27:2253-60. [PubMed]
- D’Angelo SP, Pietanza MC, Johnson ML, et al. Incidence of EGFR exon 19 deletions and L858R in tumor specimens from men and cigarette smokers with lung adenocarcinomas. J Clin Oncol 2011;29:2066-70. [PubMed]
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6. [PubMed]
- Inamura K, Takeuchi K, Togashi Y, et al. EML4-ALK fusion is linked to histological characteristics in a subset of lung cancers. J Thorac Oncol 2008;3:13-7. [PubMed]
- Martelli MP, Sozzi G, Hernandez L, et al. EML4-ALK rearrangement in non-small cell lung cancer and non-tumor lung tissues. Am J Pathol 2009;174:661-70. [PubMed]
- Yorio JT, Xie Y, Yan J, et al. Lung cancer diagnostic and treatment intervals in the United States: a health care disparity? J Thorac Oncol 2009;4:1322-30. [PubMed]
- Quarterman RL, McMillan A, Ratcliffe MB, et al. Effect of preoperative delay on prognosis for patients with early stage non-small cell lung cancer. J Thorac Cardiovasc Surg 2003;125:108-13; discussion 113-4. [PubMed]
- Bozcuk H, Martin C. Does treatment delay affect survival in non-small cell lung cancer? A retrospective analysis from a single UK centre. Lung Cancer 2001;34:243-52. [PubMed]
- Aragoneses FG, Moreno N, Leon P, et al. Influence of delays on survival in the surgical treatment of bronchogenic carcinoma. Lung Cancer 2002;36:59-63. [PubMed]
- National comprehensive cancer network. Non-small cell lung cancer: clinical practice guidelines in oncology. Available online: http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- Salloum RG, Smith TJ, Jensen GA, et al. Survival among non-small cell lung cancer patients with poor performance status after first line chemotherapy. Lung Cancer 2012;77:545-9. [PubMed]
- Halpern MT, Ward EM, Pavluck AL, et al. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncol 2008;9:222-31. [PubMed]
- Shaw AT, Yeap BY, Solomon BJ, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol 2011;12:1004-12. [PubMed]


