
HRCT features distinguishing minimally invasive adenocarcinomas from invasive adenocarcinomas appearing as mixed ground-glass nodules
Introduction
Lung cancer is considered to be the major leading cause of cancer-related mortality in the world, with lung adenocarcinoma being the most commonly encountered histological subtype of the disease (1). With the rapid advancement of imaging technologies, modern procedures, such as high-resolution computed tomography (HRCT) have made previously elusive tumors, such as numerous small-sized (<3 cm in diameter) lung adenocarcinomas, detectable by clinicians (2). Small-sized lung adenocarcinomas usually appear as pure ground glass nodules (PGGNs), mixed ground-glass nodules (MGGNs), or solid nodules; MGGNs are defined as a heterogeneous attenuation whose solid portion obscures the vascular markings underneath (3). MGGNs have a higher rate of malignancy and are of major concern due to their close association with invasive pulmonary adenocarcinomas, including both minimally invasive adenocarcinomas (MIAs) and invasive adenocarcinomas (IACs) (4). In addition, the management for MIAs and IACs tumors is different in current practice, and patients with MIAs are reported with a nearly 100% 5-year disease-free survival (DFS) rate, after a complete resection and recurrence, with lymph node metastasis being a rare occurrence. As the latest data indicates, MIAs lesions might serve as effective candidates for a sublobar resection, yet a lobectomy is the treatment of choice for IACs, which have a 74.6% 5-year DFS rate, and high recurrence and lymph node metastasis rates (5,6). Accordingly, MIAs should be accurately distinguished from IACs, appearing as MGGNs before or during surgery. This study aimed at exploring the capacity of HRCT features to discern between MIAs and IACs among MGGNs, and proposes criteria for selecting candidates for limited surgical resection.
Methods
Patients
This retrospective study was approved by the Institutional Review Board of our hospital (No. 2017056), and all of the patients involved, gave their informed consent. Patients were drawn from the Zhoushan Hospital of Zhejiang province, China, from January 2016 to December 2017. HRCT scans of 186 consecutive patients with 200 MGGNs were reviewed. The patients were aged between 23 and 81 years (average, 57.9±10.9 years) and consisted of 61 males and 125 females. Patients with MIAs were aged between 23 and 75 years (average, 55.5±11.0 years) (P>0.05), and patients with IACs and were aged between 31 and 81 years (average, 60.37±10.4 years) (P>0.05). All nodules were surgically resected and pathologically proven to be MIAs or IACs (102 MIAs and 98 IACs). Nodule-type distributions among the patience were as follows: 88 patients had 1 MIA, 4 patients had 2 MIAs, 2 patients had 2 MIAs and 1 IAC, and 2 patients had 1 MIA and 1 IAC. Eighty-seven patients had 1 IAC, 2 patients had two IACs, and 1 patient had three IACs.
For the 87 patients with single IAC nodules, wedge resection, a segmentectomy, and a lobectomy was conducted in 64, 12, and 11 patients, respectively. Meanwhile, for the 88 patients with single MIA nodules, these 3 procedures were conducted in 81, 5, and 2 patients, respectively. Among the remaining 11 enrolled patients with multiple nodules, 6 underwent lobectomy and 2 underwent segmentectomy resection with multiple nodules in the same lobe; the remaining 3 patients underwent a simultaneous lobectomy and segmentectomy resection with the nodules involving multiple lobes.
CT screening
Routine HRCT scans were performed using an Aquilion 64-detector row scanner (Toshiba, Tokyo, Japan), with a non-enhanced helical CT performed at the end of an inspiration procedure during a single breath hold with the following scanning parameters: detector collimation, 64 mm × 0.5 mm; pitch, 1.08; section thickness and interval, both 1.0 mm; scan time, 5–7 s; matrix, 512 × 512; FOV, 350 mm; 120 kVp and 350 mA. The target image was reconstructed with a section thickness and interval of 1.0 mm when a lung nodule was found.
CT imaging analysis
Two CT technologists (H Cao and S Wang) with 21 and 25 years of experience respectively, reviewed the target’s reconstruction images. The qualitative variables for each nodule were analyzed according to the following parameters: (I) lobulated shape; (II) spiculated margin; (III) air bronchogram; (IV) bubble lucency; and (V) pleural indentation. The quantitative variables included the CT values of the whole nodule, the ground glass and solid components of the MGGNs (assessed in the largest area of interest that excluded the pulmonary vessels), and the largest diameters of the nodule and solid component on axial sections. The measurements were taken 3 times by a radiologist (H Cao), and the mean values were recorded.
Histopathology
The surgically resected specimens were fixed in a 10% formalin solution, embedded in paraffin, sectioned with a microtome, and stained with hematoxylin and eosin. Histopathological analysis of the nodules was performed and classification was done using after a consensus between the two experienced lung pathologists (Z Wang and L Qian) according to the 2011 international multidisciplinary classification of lung adenocarcinoma.
Statistical analysis
We used the Pearson χ2 test to analyze the qualitative HRCT features of the MIAs and IACs appearing as MGGNs. The quantitative variables were compared using the t-test. Variables with a statistical significance in the univariate analysis were entered into a multivariate logistic regression analysis. The variables that exhibited significant differences in the multivariate logistic regression underwent receiver operating characteristic (ROC) analyses. P values <0.05 were considered significant. All statistical data were analyzed using the SPSS software program (ver. 20.0; IBM Corp., Armonk, NY, USA).
Results
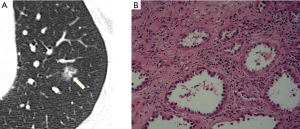
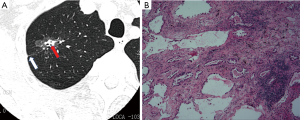
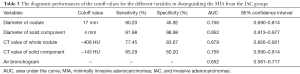
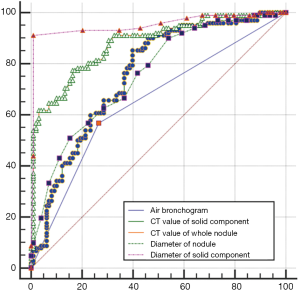
In Table 1, the HRCT morphological characteristics of lung IACs and MIAs are summarized and compared. The univariate analysis shows an evident difference (P<0.05) between the diameter of the whole nodule and the solid component of the MGGNs, and between the CT values of the whole nodule and the ground glass and solid components of the MGGNs, along with a lobulated shape, spiculated margin, air bronchogram, bubble lucency, and pleural indentation. Compared with the MIA group, the IACs exhibited larger nodule (17.7 vs. 12.4 mm, P=0.000) and solid component diameters (9.5 vs. 3.9 mm, P=0.000), higher CT values for the whole nodule (−288.15 vs. −498.66 HU, P=0.000) and ground-glass components (−440.06 vs. −594.09 HU, P=0.000) and solid components of the MGGNs (−125.83 vs. −261.12 HU, P=0.000), and greater frequencies of lobulated shape (P=0.000), spiculated margin (P=0.000), air bronchogram (P=0.000), bubble lucency (P=0.003) and pleural indentation (P=0.000) (Figures 1,2). Table 2 illustrates the results of the multivariate logistic regression analysis of the MGGNs in the MIA and IAC groups. The results showed that a larger nodule and the solid component diameters, the higher CT values of the whole nodule and solid components, and the presence of an air bronchogram were evidently related to the IACs (P=0.018, 0.001, 0.004, 0.014 and 0.006, respectively). Table 3 and Figure 3 display the summary of the cutoffs and their diagnostic performance in distinguishing the MIA and IAC groups, as determined by ROC analyses. The cutoff values of the nodule and solid component diameters were 17 mm (sensitivity: 90.20%, specificity: 45.92%) and 4 mm (sensitivity: 91.98%, specificity: 98.98%) respectively, and the cutoff CT values for the whole nodule and solid components were −408 HU (sensitivity: 77.45%, specificity: 83.67%) and −143 HU (sensitivity: 85.29%, specificity: 60.20%) respectively.
Full table
Full table
Full table
Discussion
The terms “mixed subtype adenocarcinoma” and “bronchioloalveolar carcinoma (BAC)” are no longer applied in the new international multidisciplinary classification of lung adenocarcinoma. Instead, novel concepts like adenocarcinoma in situ (AIS) and MIA have been introduced. MIA is defined as a small adenocarcinoma (≤3 cm), with a predominantly lepidic pattern and ≤5 mm invasion in the greatest dimension in any one focus (7). In this study, the optimal cutoff value for the whole nodule diameter to discriminate MIAs from IACs appearing as MGGNs was 17.0 mm, which yielded a sensitivity, specificity, and area under the curve (AUC) of 90.20%, 45.92%, and 0.756, respectively. A previous study revealed that a larger nodule diameter was independently associated with IAC in GGNs (<2 cm in diameter) at a cutoff diameter of 12.2 mm, which yielded a sensitivity, specificity, and AUC of 85.0%, 62.0%, and 0.75, respectively (8). An optimal cutoff diameter of 14.0 mm was reported that had a sensitivity of 66.7% and specificity of 73.8% for differentiating pre-invasive lesions from invasive pulmonary adenocarcinomas in MGGNs (9). Moreover, Liu et al. reported that a cutoff diameter of 12.5 mm for MIA-IACs (10) and of 11.0 mm for IACs, in PGGN had a sensitivity of 95.8% and specificity of 46.8% (11). Because our study was focused on differentiating MIAs from IACs appearing as MGGNs, it is sensible to have a different cutoff diameter from those of previous studies. Regardless, further studies of the optimal cutoff value still need to be performed.
In our study, the ROC curve had revealed that the diameter of the solid component (4.0 mm) may be the most prognostic factor in predicting IAC, yielding a sensitivity of 92.0%, a specificity of 99.0%, and an AUC of 0.952. Multivariate logistic regression analysis revealed that a smaller diameter of the solid component significantly differentiated MIAs from IACs. Zhang et al.(8) also concluded that the diameter of the solid component (6.7 mm) was the most powerful parameter for discriminating AIS-MIAs from IACs appearing as GGNs (<2 cm in diameter), with sensitivity and specificity values of 79.0% and 62.0%, respectively, and an AUC of 0.790. Cohen et al. (12) also showed that the solid component >5.0 mm in diameter had a 100.0% sensitivity and a 45.0% specificity for diagnosing invasive adenocarcinomas in Caucasian patients. Their results for IAC status showed that optimal specificity was obtained with a 9.0 mm threshold for the solid component, with a sensitivity and specificity of 70.0% and 100.0%, respectively. This study showed that the only independent feature differentiating between AIS-MIAs and IACs was the size of the solid component, which was significantly larger in the IAC group. In generally, the solid components of MGGNs are also the invasive components, or either fibrotic proliferations or collapsed alveolar spaces (13,14).
It is also suggested in our study that the mean CT values of the solid components were −126 HU for the IACs and −261 HU for the MIAs, and the above values were evidently related to IACs when the cutoff CT value was −143 HU. As Zhang et al. reported, the mean CT values of the solid components of GGNs, for MIAs and AAH-AISs were −195 and −318 HU, respectively (15). Meantime, They also reported in another two studies that the mean CT values of the solid components of GGNs were −231 HU for the AIS-MIAs and −130 HU for the IACs, and the mean CT values of the solid components of MGGNs (<2 cm in diameter) were −231 HU for the pre-invasive lesions and −195 HU for the MIAs (8,15). As our study was concerned with the discernment between MIAs and IACs appearing as MGGNs, it is reasonable that the mean CT values of the solid components differed from those of the previous studies.
Xiang et al. (16) suggested that a mean CT value less than −520 HU in the whole nodule, indicated atypical adenomatous hyperplasia (AAH) or AIS, rather than indicating MIA appearing as pure GGN measuring ≤10 mm on thin-section CT. Chae et al. (17) reported that the mean CT values of nodule that manifesting as MGGNs of pre-invasive lesions and invasive pulmonary adenocarcinomas are −574 and −440 HU, respectively. Akihiko et al. reported mean CT values of pure GGNs in IAC, MIA, AIS and AAH of −532, −509, −580, and −699 HU, respectively. All AAH lesions had values under −600 HU, and notably lower CT values than IAC, MIA, and AIS. There was significant difference between MIAs and AISs, and between invasive and noninvasive lesions (both P<0.05). Akihiko et al.’s study (18) did not observe any evident difference in the CT values between IACs and MIAs. However, our study showed that the difference in CT values between MIAs and IACs manifesting as MGGNs was significant (P<0.05); the mean CT values of nodules in MIAs and IACs were −499 and −288 HU, respectively.
In previous studies, the prevalence of an air bronchogram was significantly higher in adenocarcinoma than in squamous cell (P<0.01) or small cell carcinoma (P<0.01) (19,20), and there were significantly more invasive lesions than pre-invasive lesions appearing as GGNs on HRCT (21-23). Our findings are consistent with those of previous studies, in that the air bronchogram was associated with more invasive adenocarcinomas (43.0% of the MIAs vs. 73.0% of the IACs). As Yoshino et al. (24) suggest, an air bronchogram turns out to be a crucial independent prognostic factor, although this perspective remains controversial. Therefore, the relationship between the air bronchogram and tumor invasiveness requires further study.
Univariate analyses in previously published findings (9,24,25) revealed that a non-lobulated shape, non-spiculated margin, lack of bubble lucency, and non-pleural indentation were significantly more frequent in MIAs than IACs. Yet, in the multivariate logistic regression analyses, the above noted factors were insignificant. The results of our study are not in agreement with the studies that have investigated HRCT features differentiating tumor invasiveness from noninvasiveness. We aimed to explore HRCT features that discriminate MIAs from IACs appearing as pulmonary MGGNs, which could explain the difference in findings.
If patients with MIAs undergo sublobar resection, such as segmentectomy or wedge resection, systematic lymph node dissection (SLND) or sampling might be avoided. Because patients with MIAs have almost a 100.0% 5-year DFS rate after sublobar resection, recurrence and lymph node metastasis in these patients is rare. Comparatively, the 5-year DFS of patients carrying IACs of pathological stage IA reaches 74.6% (5,6). Due to the high rates of recurrence and lymph node metastasis, patients carrying IACs are required to take a lobectomy resection, such that SLND or sampling might be required. A mere 23 patients of a total 88 patients with resected single MIA nodules went through the sublobar resection. We propose that sublobar resection procedures should be conducted more frequently for MIAs appearing as MGGNs, and lobectomy resection procedures should be conducted more frequently for IACs appearing as MGGNs.
If HRCT features are able to be employed prior to surgery to accurately distinguish between IACs and MIAs appearing as MGGNs, appropriate surgical approaches will likely be adopted during the surgery procedure to the benefit of more patients. Our study showed that lung MIAs appearing as MGGNs frequently exhibited a solid component and nodule with the diameters of 4.0 and ≤17.0 mm, respectively, along with the CT scan values of the whole nodule and solid component of ≤−408 HU and −143 HU, respectively, as well as fewer air bronchograms. Thus, these MGGNs are suitable for sublobar resection, such that SLND and sampling can be avoided. In contrast, patients with IACs that do not exhibit these characteristics should undergo lobectomy resection, for which SLND or sampling might be performed.
A number of limitations to this study should be noted. First, our study used a retrospective design and the number of patients was relatively small. Our results should be further validated using a prospective design, including a large number of cases drawn from multiple centers. Second, because the nodules were small, some HRCT features, such as lobulated shape and pleural indentation, were less typical Finally, the heterogeneity of the results for different observers was not assessed.
In conclusion, HRCT features (air bronchogram, CT values of the whole nodule and solid component, and nodule and solid component diameters) can be used to accurately distinguish MIAs from IACs appearing as MGGNs. Our findings may provide useful guidance when selecting candidates for sublobar or lobectomy resection.
Acknowledgments
Funding: This work was supported by the Science and Technology Program of Zhoushan City (No.2017C31102).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.10.14). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study was approved by the Institutional Review Board of our hospital (No. 2017056), and all of the patients involved, gave their informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Jiang L, Yin W, Peng G, et al. Prognosis and status of lymph node involvement in patients with adenocarcinoma in situ and minimally invasive adenocarcinoma-a systematic literature review and pooled-data analysis. J Thorac Dis 2015;7:2003-9. [PubMed]
- Kitami A, Kamio Y, Hayashi S, et al. One-dimensional mean computed tomography value evaluation of ground-glass opacity on high-resolution images. Gen Thorac Cardiovasc Surg 2012;60:425-30. [Crossref] [PubMed]
- Godoy MC, Sabloff B, Naidich DP, et al. Subsolid pulmonary nodules: imaging evaluation and strategic management. Curr Opin Pulm Med 2012;18:304-12. [Crossref] [PubMed]
- Van Schil PE, Asamura H, Rusch VW, et al. Surgical implications of the new IASLC/ATS/ERS adenocarcinoma classification. Eur Respir J 2012;39:478-86. [Crossref] [PubMed]
- Zhang J, Wu J, Tan Q, et al. Why do pathological stage IA lung adenocarcinomas vary from prognosis? A clinicopathologic study of 176 patients with pathological stage IA lung adenocarcinoma based on the IASLC/ATS/ERS classification. J Thorac Oncol 2013;8:1196-202. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-285. [Crossref] [PubMed]
- Zhang Y, Shen Y, Qiang JW, et al. HRCT features distinguishing pre-invasive from invasive pulmonary adenocarcinoma appearing as ground-glass nodules. Eur Radiol 2016;26:2921-8. [Crossref] [PubMed]
- Lee SM, Park CM, Goo JM, et al. Invasive pulmonary adenocarcinomas versus preinvasive lesions appearing as ground-glass nodules: differentiation by using CT features. Radiology 2013;268:265-73. [Crossref] [PubMed]
- Liu LH, Liu M, Wei R, et al. CT findings of persistent pure ground glass opacity: can we predict the invasiveness? Asian Pac J Cancer Prev 2015;16:1925-8. [Crossref] [PubMed]
- Eguchi T, Yoshizawa A, Kawakami S, et al. Tumor size and computed tomography attenuation of pulmonary pure ground-glass nodules are useful for predicting pathological invasiveness. PLoS ONE 2014;9:e97867 [Crossref] [PubMed]
- Cohen JG, Reymond E, Lederlin M, et al. Differentiating pre- and minimally invasive from invasive adenocarcinoma using CT features in persistent pulmonary part-solid nodules in Caucasian patients. Eur J Radiol 2015;84:738-44. [Crossref] [PubMed]
- Noguchi M, Morikawa A, Kawasaki M, et al. Small adenocarcinoma of the lung - histologic characteristics and prognosis. Cancer 1995;75:2844-52. [Crossref] [PubMed]
- Park CM, Goo JM, Lee HJ, et al. Nodular ground-glass opacity at thin-section CT: histologic correlation and evaluation of change at follow up. RadioGraphics 2007;27:391-408. [Crossref] [PubMed]
- Zhang Y, Qiang JW, Ye JD, et al. High resolution CT in differentiating minimally invasive component in early lung adenocarcinoma. Lung Cancer 2014;84:236-41. [Crossref] [PubMed]
- Xiang W, Xing Y, Jiang S, et al. Morphological factors differentiating between early lung adenocarcinomas appearing as pure ground-glass nodules measuring ≤ 10 mm on thin-section computed tomography. Cancer Imaging 2014;14:33. [Crossref] [PubMed]
- Chae HD, Park CM, Park SJ, et al. Computerized Texture analysis of Persistent Part-solid ground-glass nodules: Differentiation of Preinvasive Lesions from Invasive Pulmonary Adenocarcinomas. Radiology 2014;273:285-93. [Crossref] [PubMed]
- Chae HD, Park CM, Park SJ, et al. Correlation between histological invasiveness and the computed tomography value in pure ground glass nodules. Radiology 2014;273:285-93. [Crossref] [PubMed]
- Cui Y, Ma DQ, Liu WH. Value of multiplanar reconstruction in MSCT in demonstrating the relationship between solitary pulmonary nodule and bronchus. Clin Imaging 2009;33:15-21. [Crossref] [PubMed]
- Jiang B, Takashima S, Miyake C, et al. Thin-section CT findingsin peripheral lung cancer of 3 cm or smaller: are there any characteristic features for predicting tumor histology or do they depend only on tumor size? Acta Radiol 2014;55:302-8. [Crossref] [PubMed]
- Zhang Y, Qiang JW, Shen YU, et al. Using air bronchograms on multi-detector CT to predict the invasiveness of small lung adenocarcinoma. Eur J Radiol 2016;85:571-7. [Crossref] [PubMed]
- Lim HJ, Ahn S, Lee KS, et al. Persistent pure ground-glass opacity lung nodules ≥ 10 mm in diameter at CT scan: histopathologic comparisons and prognostic implications. Chest 2013;144:1291-9. [Crossref] [PubMed]
- Oda S, Awai K, Liu D, et al. Ground-glass opacities on thin-section helical CT: differentiation between bronchioloalveolar carcinoma and atypical adenomatous hyperplasia. AJR Am J Roentgenol 2008;190:1363-8. [Crossref] [PubMed]
- Yoshino I, Nakanishi R, Kodate M, et al. Pleural retraction and intra-tumoral air-bronchogram as prognostic factors for stage I pulmonary adenocarcinoma following complete resection. Int Surg 2000;85:105-12. [PubMed]
- Liu LH, Liu M, Wei R, et al. CT Findings of Persistent Pure Ground Glass Opacity Can We Predict the Invasiveness? Asian Pac J Cancer Prev 2015;16:1925-8. [Crossref] [PubMed]


