
Prospective analysis of liquid biopsies of advanced non-small cell lung cancer patients after progression to targeted therapies using GeneReader NGS platform
Introduction
Acquired resistance is an unavoidable process during treatment of cancer patients with targeted therapies. In the case of first and second generation EGFR-TKIs, EGFR mutant (EGFR-mut) non-small cell lung cancer (NSCLC) patients usually progress after a median period of 10–12 months (1). The molecular mechanisms responsible for this process have been extensively investigated and the T790M secondary mutation has emerged as the most frequent resistance-associated molecular alteration in EGFR-mut patients, with prevalence around 50–60% (1-3), followed by amplification of membrane receptors, such as MET proto-oncogene (MET), erb-b2 receptor tyrosine kinase 2 (ERBB2) or fibroblast growth factor receptor 1 (FGFR1) (4-7). Other resistance mechanisms include epithelial to mesenchymal transformation (EMT) or histological transformation to small cell lung cancer (SCLC) (4). Third generation EGFR-TKIs are active against tumors with the T790M secondary mutation and one of them, osimertinib, has been approved in this setting (4). However, similarly to first and second line TKIs, patients ultimately progress. Mechanisms of resistance to osimertinib include loss of the T790M (5), emergence of a “tertiary” mutation in exon 20 of EGFR (C797S) (8-11), MET and ERBB2 amplifications and de novo mutations in KRAS (6,12).
In the case of EML4-ALK positive patients, disease progression occurs after a median of 9–18 months of treatment with ALK-TKIs (13-17). Different mechanisms of acquired resistance have been identified including emergence of ALK secondary mutations, which can be detected in 30% of patients, activation of the EGFR signaling pathway, KRAS mutations and others (18,19). Finally, EMT and SCLC transformation have also been described in this setting (20,21). In contrast with EGFR, the spectrum of ALK mutations associated with resistance to ALK-TKIs is very heterogeneous, being the most common L1196M, G1269A, F1174LC/L, C1156Y and G1202R (22). Importantly, second and third generation ALK-TKIs show different efficacies depending on the type of secondary mutation (23).
Genetic analysis of somatic alterations is mandatory in advanced NSCLC at presentation and progression to targeted agents, in order to select the optimal treatment strategy. However, around 5–20% of patients cannot be biopsied baseline or the tumor tissue in biopsies or cytological samples is insufficient for successful genetic analysis (24,25). This percentage is significantly higher in patients progressing to targeted therapies, where the availability of rebiopsies is limited. In consequence, liquid biopsy samples are of particular relevance in this setting as a surrogate of surgical biopsies for genetic testing (26).
Here, we present the results of the NGS analysis in liquid biopsy samples of NSCLC patients obtained at progression to different therapies using the GeneReadTM QIAact Lung DNA UMI Panel. Our results demonstrate the usefulness of NGS for the detection of genetic alterations associated with resistance in liquid biopsies and, consequently, for the selection of subsequent lines of therapy.
Methods
Sample selection and processing
For the validation of the panel, a total of 45 samples were retrospectively analyzed. They included 20 FFPE samples, 10 paired FFPE/blood samples and 5 cell lines included in paraffin blocks. Patient samples comprised a majority of NSCLC (n=27/30; 90%), but also colorectal (n=1), melanoma (n=1) and ovary (n=1) specimens. All blood and tissue samples had been previously genotyped by non-NGS methodologies, namely PNA-Q-PCR (27), Sanger sequencing or FISH, and were selected to represent a variety of clinically relevant mutations.
For prospective analysis, liquid biopsies of 24 NSCLC patients were obtained at progression to different therapies. They included peripheral blood (n=18, two patients with paired blood-other fluids), pleural fluid (PF, n=3), cerebrospinal fluid (CSF, n=4) and ascites (AF, n=1). Peripheral blood (10 mL) was collected in Vacutainer tubes (BD, Plymouth, UK) and centrifuged at 2,300 rpm for 10 min. The supernatant was then transferred into a new tube and submitted to a second centrifugation immediately followed by cfDNA purification or storage at −20 °C. Other fluids (1–10 mL) were processed following the same protocol, cytological extensions of the first and second pellets (sometimes not visible) were performed to evaluate positivity for malignant cells and absence of remaining cells in the final preparation, respectively.
Studies were conducted in accordance with the Declaration of Helsinki under an approved protocol of the institutional review board of the Quirón Hospitals, and de-identified for patient confidentiality. Informed written consent was obtained from all subjects.
cfDNA purification
Purification of cfDNA was performed from 4 mL of fluids using a custom protocol with the QIAsymphony® DSP Virus/Pathogen Midi Kit using a QIAsymphony robot (QIAGEN, Hilden, Germany) and following the manufacturer’s instructions. The final elution volume was 50 µL per sample. For liquid biopsies with less than 4 mL, an alternative custom protocol using 1.2 mL and a final elution volume was 30 µL was used. For DNA purification from FFPE samples, the GeneRead DNA FFPE Kit (QIAGEN, Hilden, Germany) was employed, following the manufacturer’s instructions. DNA concentration was measured by Qubit®. Samples with ≥2.5 ng DNA/mL were diluted to achieve this concentration.
NGS sample preparation, sequencing run and data processing
NGS was performed with the GeneReader Platform® (QIAGEN, Hilden, Germany). Purified DNA (16.75 µL) was used as a template to generate libraries for sequencing using the GeneReadTM QIAact Lung DNA UMI Panel, according to manufacturer’s instructions. The panel is designed to enrich specific target regions containing 550 variant positions in 19 selected genes frequently altered in lung cancer tumors (AKT1, ALK, BRAF, DDR2, EGFR, ERBB2/HER2, ESR1, KIT, KRAS, MAP2K1, MET, NRAS, NTRK1, PDGFRA, PIK3CA, PTEN, ROS1, FGFR1 and RICTOR), including MET exon 14 skipping mutations. The panel can also detect copy number variations (CNV) in five genes (EGFR, FGFR1, ERBB2/HER2, MET, RICTOR).
Libraries were quantified using a QIAxcel® Advanced System, diluted to 100 pg/µL and pooled in batches of 6 (liquid biopsies) or 12 (tissues). Clonal amplification was performed on 625 pg of pooled libraries by the GeneRead Clonal Amp Q Kit using the GeneRead QIAcube and an automated protocol. Following bead enrichment, pooled libraries were sequenced using the GeneRead UMI Advanced Sequencing Q kit in a GeneReader instrument.
QIAGEN Clinical Insight Analyze (QCI-A) software was used to performed the secondary analysis of FASTQ reads, align the read data to the hg19 reference genome sequence, call sequence variants and generate a report for visualization of the sequencing results. Variants were imported into the QIAGEN Clinical Insight Interpret (QCI-I) web interface for data interpretation and generation of final custom report.
Results
Validation of the panel
The NGS workflow using GeneReader requires approximately 5 days from DNA extraction to a final clinical report and allows processing of up to 36 samples per run, with relatively low hands-on time (Figure 1). In order to implement the GeneReader Platform together with the QIAact Lung DNA Panel in routine clinical practice, a retrospective validation study was performed. A total of 45 clinical samples, previously genotyped by other methodologies, were selected for the validation cohort, including FFPE tumor tissues (n=20), cell lines embedded in paraffin (n=5) and paired FFPE/blood samples (n=10 each).
Of the 20 FFPE tumor tissues included in the validation cohort, 11 (55%) were biopsies at diagnostic and 9 (45%) at the time of progression. Concordant results with previous hotspot EGFR, KRAS and BRAF mutation analysis were obtained in all cases (Table 1). In sample 15, NGS detected two previously unrecognized non-V600 BRAF mutations, p.G469A in exon 11 and a rare p.W604C mutation in exon 15; while in sample 10, corresponding to a patient progressing to EGFR-TKIs, a resistance EGFR p.G742S mutation in exon 18 was found. The three non-hotspot mutations were confirmed by Sanger sequencing. Finally, sample 4 harbored a 12 pb insertion in ERBB2 gene (exon 20) that is not included in the GeneReadTM QIAact Lung DNA panel and was therefore not detected by NGS. Regarding CNV analysis, most copy number gains apparent by NGS were confirmed by FISH. They included ERBB2 (progression, patient 9), EGFR (baseline, CRC patient 14) and MET gene (progression, patient 6) amplifications; but also concomitant CNVs in re-biopsies of five EGFR mutant patients at progression, EGFR and ERBB2 in patients 8 and 10, and EGFR and MET in patient 12. The only discordant case was the patient 13, where MET amplification was not confirmed by FISH. Finally, in the five cell lines tested, the results of the NGS analyses were fully concordant with the previous genotyping both for mutations and copy number variations. The only exception was the cell line NCI-H1781, which harbors a 3pb insertion in the exon 20 of ERBB2 gene that is not included in the GeneReadTM QIAact Lung DNA panel and therefore could not be detected by NGS.
Table 1
| Sample | Cancer type | Type of sample | Region of pathogenic variants | Gene | Exon | Mutation (amino acid change) | VAF mutation (%) | Concordance (mutations) with previous genotyping | CNVs analysis | Concordance (CNVs) with previous genotyping |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Lung | B | 55259514..55259515 | EGFR | 21 | p. L858R | 15 | Yes | No detected | Yes |
| 2 | Lung | B | 25398284 | KRAS | 2 | p. G12V | 46.61 | Yes | No detected | Yes |
| 3 | Lung | B | – | – | – | No pathogenic alterations | – | Yes | No detected | Yes |
| 4 | Ovary | B | – | – | – | No pathogenic alterations | – | Yes* | No detected | Yes |
| 5 | Lung | P | 55242468..55242479 | EGFR | 19 | p.L747_T751delinsP | 21.32 | Yes | EGFR amplification | Yes |
| 6 | Lung | P | 55259515 | EGFR | 21 | p. L858R | 9.69 | Yes | MET amplification | Yes |
| 7 | Lung | P | 55259515 | EGFR | 21 | p. L858R | 4.65 | Yes | No detected | Yes |
| 8 | Lung | P | 55259515 | EGFR | 21 | p. L858R | 6.29 | Yes | EGFR and ERBB2 amplification | Yes |
| 9 | Lung | P | 55242465..55242479 | EGFR | 19 | p. E746_A750del | 19.5 | Yes | ERBB2 amplification | Yes |
| 10 | Lung | P | 55242467..55242485 | EGFR | 19 | p. E746_S752delinsV | 31.72 | Yes | EGFR and ERBB2 amplification | Yes |
| 55241722 | EGFR | 18 | p. G742S | 14.84 | ||||||
| 11 | Lung | P | 55242465..55242479 | EGFR | 19 | p. E746_A750del | 10.74 | Yes | No detected | Yes |
| 12 | Lung | P | 55259515 | EGFR | 21 | p. L858R | 46 | Yes | EGFR and MET amplification | Yes |
| 13 | Lung | P | 55259515 | EGFR | 21 | p. L858R | 40.3 | Yes | EGFR and MET amplification | No** |
| 14 | CRC | B | 25398284 | KRAS | 2 | p. G12V | 47.08 | Yes | EGFR amplification | Yes |
| 15 | Lung | B | 140481402 | BRAF | 11 | p. G469A | 34.29 | Yes | No detected | Yes |
| 140453123 | BRAF | 15 | p. W604C | 37.46 | ||||||
| 16 | Lung | B | 55259515 | EGFR | 21 | p. L858R | 8.79 | Yes | No detected | Yes |
| 17 | Lung | B | 140453136 | BRAF | 15 | p. V600E | 31.65 | Yes | No detected | Yes |
| 18 | Lung | B | – | – | – | No pathogenic alterations | – | Yes | No detected | Yes |
| 19 | Lung | B | 25398281 | KRAS | 2 | p. G13D | 34.03 | Yes | No detected | Yes |
| 20 | Lung | B | 25398284 | KRAS | 2 | p. G12D | 31.2 | Yes | No detected | Yes |
| NCI-H1975 | Lung | CL | 55259515 | EGFR | 21 | p. L858R | 78.3 | Yes | No detected | Yes |
| 55249071 | EGFR | 20 | p. T790M | 76.3 | ||||||
| Hs 746T | Stomach | CL | 116412044 | MET | 14 | c.3082+1G>T (splicing variant) | 99 | Yes | ERBB2 and MET amplification | Yes |
| NCI-H1781 | Lung | CL | – | – | – | No pathogenic alterations | – | Yes* | No detected | Yes |
| EBC-1 | Lung | CL | – | – | – | No pathogenic alterations | – | Yes | MET amplification | Yes |
| NCI-H596 | Lung | CL | 178936091 | PIK3CA | 10 | p. E545K | 52.3 | Yes | EGFR amplification | Yes |
| 55249071 | MET | 14 | c.3082+1G>T (splicing variant) | 95 |
*, patient 4 and cell line NCI-H1781 presents exon 20 ERBB2 insertions (p.G776>VC and p.Y772_A775dup respectively), not included in the panel; **, discordant result for MET amplification between NGS (positive) and FISH (negative). VAF, variant allelic fraction; CNV, copy number variation; B, baseline. P, progression. CL, cell line.
Concordant results in NGS mutation analysis were obtained for all FFPE (n=10) and plasma (n=10) paired samples (Table 2). Single mutations in EGFR or BRAF genes were identified in six of these paired DNAs, while one pair harbored concomitant mutations in EGFR and PIK3CA. Finally, three samples pairs were wt for all genes in the NGS panel. The mutation allelic fractions (VAFs) determined by NGS in tissue were higher than the VAF in paired plasma samples, with the only exception of patient four. NGS also detected EGFR amplification in four EGFR-mutant FFPE samples. Of those, only patient four was also positive in paired plasma.
Table 2
| Sample | Cancer type | Type of sample | Region of pathogenic variants | Gene | Exon | Mutation (amino acid change) | VAF mutation (%) in FFPE | VAF mutation (%) in plasma | Concordance (mutations) with previous genotyping | CNVs analysis (FFPE) | CNVs analysis (plasma) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Lung | B | 178952085 | PIK3CA | 21 | p.H1047R | 49,42 | 5.3 | Yes | No detected | No detected |
| 55249010^55249011 | EGFR | 20 | p. N771_H773dup | 27,49 | 3.48 | ||||||
| 2 | Lung | B | 140481402 | BRAF | 11 | p. G469A | 21.13 | 2.67 | Yes | No detected | No detected |
| 3 | Lung | B | 55242465..55242479 | EGFR | 19 | p. E746_A750del | 19.53 | 0.51 | Yes | EGFR amplification | No detected |
| 4 | Lung | B | 55249005^55249006 | EGFR | 20 | p. V769_D770insGTV | 31.02 | 59.8 | Yes | EGFR amplification | EGFR amplification |
| 5 | Lung | B | 55249010^55249011 | EGFR | 20 | p. N771_H773dup | 59.65 | 4.0 | Yes | EGFR amplification | No detected |
| 6 | Melanoma | B | 140453136 | BRAF | 15 | V600E | 22.71 | 14 | Yes | No detected | No detected |
| 7 | Lung | B | – | – | – | No pathogenic alterations | – | – | Yes | No detected | No detected |
| 8 | Lung | B | – | – | – | No pathogenic alterations | – | – | Yes | No detected | No detected |
| 9 | Lung | B | 55259515 | EGFR | 21 | p. L858R | 46.15 | 7.4 | Yes | EGFR amplification | No detected |
| 10 | Lung | B | – | – | – | No pathogenic alterations | – | – | Yes | No detected | No detected |
VAF, variant allelic fraction; CNV, copy number variation; B, baseline.
Prospective analysis of liquid biopsies and clinical characteristics of patients
From December 2017 to June 2018, we prospectively analyzed liquid biopsy samples from 24 advanced NSCLC patients progressing to different therapies, including 13 EGFR-mut patients treated with first and third generation EGFR-TKIs and nine EML4-ALK-positive patients relapsing to several ALK-TKIs. None of the 24 patients had a re-biopsy available for molecular analysis at the time to progression (Table 3). Of the 13 EGFR-mut patients, seven harbored exon 19 deletions at presentation, three exon 20 insertions and three the exon 21-p.L858R point mutation. Regarding the nine ALK-positive patients, the specific ALK-EML4 variant at presentation had been determined in five and was unknown in four. Finally, two BRAF-mut patients progressing to chemotherapy and anti-PD-L1 treatment were included in the study.
Table 3
| Characteristics | Total of patients (N=24) |
|---|---|
| Gender | |
| Male | 10 (41.7%) |
| Female | 14 (58.3%) |
| Smoking status | |
| Never smokers | 14 (58.3%) |
| Former smokers | 9 (37.5%) |
| Smokers | 1 (4.2%) |
| Histology | |
| Adenocarcinoma | 20 (83%) |
| NSCLC (NOS) | 4 (17%) |
| Type of progression | |
| Extracranial oligometastases | 9 (37.5%) |
| CNS progression | 5 (20.8%) |
| Systemic progression | 4 (16.7%) |
| Unknown | 6 (25.0%) |
| Type of treatment | |
| First or second-generation EGFR TKI | 7 (29.2%) |
| Third-generation EGFR TKI | 6 (25.0%) |
| ALK inhibitors | 9 (37.5%) |
| Other targeted therapies | 2 (8.3%) |
CNS, central nervous system; NSCLC, non-small cell lung cancer; NOS, not otherwise specified.
At the time to progression, 9/24 patients (37.5%) had developed a limited number of metastatic extracranial oligometastases in lung (n=4), liver (n=2) or pleura (n=3), while 5/24 patients (20.8%) showed metastases exclusively in the central nervous system (CNS) and 4/24 patients (16.7%) presented a systemic progression. Finally, data about metastatic sites was unavailable in six cases. CSF was collected in all patients with CNS progression, with the only exception of patient 17, where lumbar puncture was not feasible and plasma was used instead. Similarly, PF was analyzed in 2 of the 3 patients with pleural progression. Interestingly, paired plasma and non-blood fluids (PF or CSF) were simultaneously collected in two patients (11 and 22).
Genetic alterations in liquid biopsies at progression to targeted therapies
Liquid biopsies at progression were analyzed by NGS using the QIAact Lung DNA UMI Panel in order to identify genetic alterations associated with acquired resistance to targeted therapies. The results of the analysis are summarized in Table 4. Among the seven EGFR-mut patients progressing to first generation EGFR-TKIs, NGS detected the primary sensitizing mutation in 5 (71.4%). In contrast, the secondary p.T790M resistance mutation did not appear in any case, a result that was confirmed by PNA-Q-PCR (data not shown) (27). However, other genetic alterations potentially associated with the emergence of resistance to TKIs were detected, including MET amplification in one patient and a p.L833V mutation in exon 21 of the EGFR gene in patient 7. Re-analysis of the initial biopsy demonstrated that this mutation was not present at presentation (data not shown). Interestingly, the VAFs of the sensitizing p.L858R and the potentially resistant p.L833V mutations in the plasma sample at progression were very similar (0.84% and 0.89% respectively).
Table 4
| Progression to | Patient Nº | Type of sample | Treatment | Gene | Exon | Mutation (amino acid change) | VAF mutation (%) | CNVs analysis |
|---|---|---|---|---|---|---|---|---|
| Progression to first- or second-generation TKI | 1 | P | Erlotinib | EGFR | 20 | p.A767_V769dup | 26% | EGFR amplification |
| 2 | P | Erlotinib | No mutation detected | EGFR amplification | ||||
| 3 | P | Afatinib | EGFR | 20 | p.V769_D770insGTV | 45.59% | EGFR amplification | |
| 4 | P | Erlotinib | No mutation detected | No CNVs detected | ||||
| 5 | PF | Gefitinib | EGFR | 21 | p.L858R | 13% | EGFR and MET amplification | |
| 6 | PF | Erlotinib | EGFR | 19 | p.T751_E758delTSPKANKE | 95% | EGFR amplification | |
| 7 | P | Erlotinib | EGFR | 21 | p.L858R + p.L833V& | 0.89% + 0.84%& | No CNVs detected | |
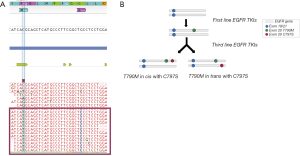
| Progression to third-generation TKI | 8 | CSF | Osimertinib | EGFR | 19, 20 | p.E746_A750del + p.T790M& + p.C797S& (*) | 6.87% + 5.14%& + 0.65%& | No CNVs detected |
| 9 | CSF | Osimertinib | EGFR | 19, 20 | p.E746_A750del + p.T790M& + p.C797S& (*) | 16.69% + 16.53%& + 11.21%& | EGFR amplification | |
| 10 | P | Osimertinib | EGFR | 19, 20 | p.E746_A750del + p.T790M& + p.C797S& (*) | 15.76% + 3.59%& + 4.77%& | EGFR and MET amplification | |
| 11 | P | Osimertinib | EGFR | 19 | p.T751_E758del | 1.56% | No CNVs detected | |
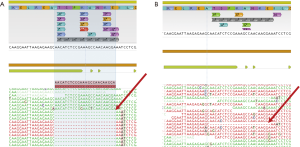
| PF | 19 | p.T751_E758del (**) | <0.1% | |||||
| 12 | P | Osimertinib | EGFR | 21 | p.L858R | 26% | EGFR and MET amplification | |
| 13 | P | Osimertinib | No mutation detected | No CNVs detected | ||||
| Progression to ALK inhibitors | 14 | CSF | Alectinib | ALK + PIK3CA | 23 + 8 | p.F1174L + p.F1174C + p.C420R& | 3.27%& + 4.03%& + 7.59%& | No CNVs detected |
| 15 | P | Crizotinib | KRAS | 2 | p.G12V& | 0.81%& | No CNVs detected | |
| 16 | P | Brigatinib | ALK + EGFR | 22 + 19 | p.I1171N + p.P741L& | 3.17% + 8.10% | EGFR amplification | |
| 17 | P | Crizotinib | No mutation detected | No CNVs detected | ||||
| 18 | P | Crizotinib | No mutation detected | No CNVs detected | ||||
| 19 | P | Lorlatinib | No mutation detected | No CNVs detected | ||||
| 20 | P | Alectinib | ALK | 25 | p.G1269A& | 0.5%& | No CNVs detected | |
| 21 | P | Brigatinib | ALK | 23 | p.F1174L& | 1.38%& | EGFR amplification | |
| 22 | P | Brigatinib | Invalid results | No CNVs evaluable | ||||
| CSF | Invalid results | No CNVs evaluable | ||||||
| Progression to other targeted therapies | 23 | P | Carboplatin-pemetrexed | BRAF | 11 | p.G469V | 2.87% | No CNVs detected |
| 24 | AF | Ipilimumab-nivolumab | BRAF | 15 | p.V600E | 19.34% | EGFR amplification |
&, mutations associated with resistance; *, patients 8, 9 and 10: p.T790M and p.C797S resistance mutations were observed in CIS configuration (for more details see Figure 2); **, patient 11: the p.T751_E758del in exon 19 was observed in PF only with manual inspection (for more details see Figure 3). PF, pleural fluid; CSF, cerebrospinal fluid; AF, ascites; VAF, variant allelic fraction; CNV, copy number variations.
In the group of six patients progressing to osimertinib, the p.C797S resistance mutation appeared in 3 (50%), accompanied in all cases by the initial EGFR sensitizing mutation and the p.T790M. Visual inspection of the reads revealed that the p.C797S and the p.T790M were in cis configuration in all cases (Figure 2). In the remaining three patients, only the sensitizing mutation in EGFR could be detected. The high allelic fractions observed in two cases strongly suggest disappearance of the p.T790M as a mechanism of resistance. Regarding CNVs, concomitant EGFR and MET amplification was detected in two patients. Interestingly, paired plasma and pleural fluid samples were available for patient 11. The sensitizing EGFR exon 19 deletion was detected with greater reliability and higher VAF in plasma. This patient was undergoing a systemic progression disease with multiple metastatic sites besides pleura (Figure 3).
Among the 9 patients progressed to ALK-TKIs, mutations in exons 22, 23 or 25 of ALK associated with acquired resistance were detected in 5 (55.6%), while a KRAS p.G12V mutation emerged in one. Finally, MET amplification was not detected in any case, but EGFR amplifications were found in two patients, both harboring ALK resistance mutations. In Patient 17, no alterations were detected by NGS in the plasma analysis. The negative results could be due to the fact that, despite a CNS progression, lumbar puncture was not feasible and only plasma could be analyzed. Invalid results were obtained for patient 22, due to a low amount of purified cfDNA. This patient presented CNS progression, but the size of the metastases was very small and they were located in a limited area of the brain.
Finally, two patients (23 and 24) progressing to therapies other than TKIs were included in the study. Patient 23 presented the BRAF p.G469V at diagnostic, which reappeared in plasma with the progression of the disease. No other mutations or CNVs were observed. Patient 24 presented a BRAF mutation at diagnostic (p.V600E), which could be detected in ascites after systemic progression to immunotherapy, together with de novo EGFR amplification
Discussion
In this study, we present the results obtained in our hospital after the implementation of the GeneReader NGS platform for the routine analysis of liquid biopsies in NSCLC patients progressing to targeted therapies. Liquid biopsy samples are of particular relevance in this setting since, although a significant number of patients cannot be re-biopsied, genetic testing is recommended to select subsequent lines of treatment or to enroll patients in appropriate clinical trials (28). At this respect, several studies have demonstrated the utility of T790M analysis in blood to select patients for osimertinib, a procedure that permits to avoid unnecessary biopsies (29). In our case, re-biopsies could not be obtained in any case and liquid biopsies were the only samples available for testing.
Mechanisms of resistance EGFR and ALK-TKIs include secondary and “tertiary” mutations in the exons coding the tyrosine kinase domains of these receptors; amplification of MET or ERBB2 and acquired mutations in PIK3CA or KRAS (30). All of these alterations can be detected by the NGS panel used in our study. Of the 21 patients progressing to TKIs with valid results in liquid biopsy, NGS analysis identified a potential mechanism of resistance in 12 (57%). Similarly to the literature, the most common acquired alterations detected in our study were secondary and “tertiary” mutations in ALK and EGFR, which appeared in 8/21 patients (38%), followed by amplifications in 5/21 patients (24%), and KRAS mutations in one patient (5%). Finally, we identified loss of the p.T790M in two patients progressing to osimertinib. Amplification of EGFR is frequently associated with EGFR sensitizing mutations baseline (31,32) and re-appears, together with them, in liquid biopsies after progression. Consequently, amplification of MET was considered a potential mechanism of resistance in all cases but EGFR copy gains only in patients progressing to ALK-TKIs, where EGFR pathway activation has been described to emerge only after relapse (33,34). Mechanisms of resistance have been described to co-occur in a significant percentage of patients progressing to targeted therapies. In our study, concomitant alterations associated with resistance could be identified in 3 of the 21 liquid biopsies (14%), namely a C797S mutation together with a MET amplification in a patient progressing to osimertinib, and secondary ALK mutations together with EGFR amplification in two patients relapsing to ALK-TKIs.
The T790M was not detected in any of the seven liquid biopsies corresponding to patients progressing to erlotinib and gefitinib. In two cases, the initial EGFR sensitizing mutation did not appear either and the presence of the T790M in tumor tissue could not be ruled out. In one patient relapsing to gefitinib, NGS detected a rare mutation in exon 21 of EGFR (p.L833V), which has been associated with resistance to EGFR-TKIs (35). In the case of patients progressing to osimertinib, we found the “tertiary” resistance mutation C797S in three of six liquid biopsies (50%). The mutation was in “cis” configuration with the T790M in all cases. Only NGS platforms can differentiate the “cis” vs. “trans” configuration, which is clinically relevant in order to determine whether the patient can be re-challenged with EGFR-TKIs (8,36,37). Loss of T790M was identified in two additional cases of patients in progression to osimertinib, where the EGFR sensitizing mutations reappeared at allelic fractions >1% but the T790M was not detected. Finally, mutations in the exons coding for the tyrosine kinase domain of ALK were identified in 4 of 8 patients (50%) progressing to ALK-TKIs. One of them presented simultaneously with two ALK resistance mutations and a PIK3CA mutation (p.C420R) that has been described as oncogenic in cell models (38), and another showed the p.I1171N resistance mutation in ALK and a mutation of uncertain significance in the exon 19 of EGFR (p.P741L). Unfortunately, samples at presentation were not available to determine if these mutations in PIK3CA and EGFR were also associated with acquisition or resistance. Finally, a p.G12V mutation in KRAS was found in a patient progressing to crizotinib. This particular mutation has already been reported as a mechanism of acquired resistance in translocated ALK patients treated with this drug (39,40).
The published studies on genetic testing by NGS in liquid biopsies have been generally limited to cfDNA isolated from plasma. In contrast, we have included eight fluids other than blood in our report. Four of them were cerebrospinal fluids of patients with CNS progressions; three were pleural fluids and one ascites. Remarkably, with the only exception of one sample with invalid results, we could detect genetic alterations in all of them, namely sensitizing mutations, resistance mutations and/or copy number alterations. The ascites sample was from a patient with rapid progression to immunotherapy and showed EGFR amplification, a genetic aberration that has been associated with hyperprogression to anti-PD-1/PD-L1 agents (41). Taken together, our results suggest that, depending on the site of progression, fluids other than blood can be used for cfDNA purification and subsequent NGS analysis.
Our study also had some limitations, some of them inherent to NGS techniques. First, some mechanisms associated with resistance could not be detected, such as EMT or SCLC transformation. Second, as discussed above, we found some mutations of uncertain significance. Third, due to the lack of paired tumor biopsies, the amplifications detected by NGS could not be validated by the gold standard technique, FISH. However, the percentage of liquid biopsies baseline positive for copy number gains by NGS (Table 2) was only 10%, compared to 24% after progression; suggesting that the amplifications determined by NGS were not false positives.
In summary, our study demonstrates that NGS can be implemented in routine clinical practice for the genetic analysis of liquid biopsy samples in patients progressing to targeted therapies. NGS can detect most of mechanisms associated with acquired resistance and provide useful information of the selection of second and subsequent lines of treatment.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Umberto Malapelle, Christian Rolfo) for the series “Targeted Therapy and Non-Small Cell Lung Cancer: A New Era?” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.10.12). The series “Targeted Therapy and Non-Small Cell Lung Cancer: A New Era?” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Studies were conducted in accordance with the Declaration of Helsinki (as revised in 2013) under an approved protocol of the institutional review board of the Quirón Hospitals, and de-identified for patient confidentiality. Informed written consent was obtained from all subjects.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26. [Crossref] [PubMed]
- Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 2013;19:2240-7. [Crossref] [PubMed]
- Costa C, Molina MA, Drozdowskyj A, et al. The impact of EGFR T790M mutations and BIM mRNA expression on outcome in patients with EGFR-mutant NSCLC treated with erlotinib or chemotherapy in the randomized phase III EURTAC trial. Clin Cancer Res ;20:2001-10. [Crossref] [PubMed]
- Tan CS, Gilligan D, Pacey S. Treatment approaches for EGFR-inhibitor-resistant patients with non-small-cell lung cancer. Lancet Oncol 2015;16:e447-59. [Crossref] [PubMed]
- Xie S, Li Y, Li X, et al. Mer receptor tyrosine kinase is frequently overexpressed in human non-small cell lung cancer, confirming resistance to erlotinib. Oncotarget 2015;6:9206-19. [Crossref] [PubMed]
- Koch H, Busto ME, Kramer K, et al. Chemical Proteomics Uncovers EPHA2 as a Mechanism of Acquired Resistance to Small Molecule EGFR Kinase Inhibition. J Proteome Res 2015;14:2617-25. [Crossref] [PubMed]
- Zhang Z, Lee JC, Lin L, et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat Genet 2012;44:852-60. [Crossref] [PubMed]
- Thress KS, Paweletz CP, Felip E, et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med 2015;21:560-2. [Crossref] [PubMed]
- Oxnard GR, Thress KS, Alden RS, et al. Association Between Plasma Genotyping and Outcomes of Treatment With Osimertinib (AZD9291) in Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:3375-82. [Crossref] [PubMed]
- Thress KS, Brant R, Carr TH, et al. EGFR mutation detection in ctDNA from NSCLC patient plasma: A cross-platform comparison of leading technologies to support the clinical development of AZD9291. Lung Cancer 2015;90:509-15. [Crossref] [PubMed]
- Fan W, Tang Z, Yin L, et al. MET-independent lung cancer cells evading EGFR kinase inhibitors are therapeutically susceptible to BH3 mimetic agents. Cancer Res 2011;71:4494-505. [Crossref] [PubMed]
- Lee HJ, Zhuang G, Cao Y, et al. Drug Resistance via Feedback Activation of Stat3 in Oncogene-Addicted Cancer Cells. Cancer Cell 2014;26:207-21. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Yoshida T, Oya Y, Tanaka K, et al. Differential Crizotinib Response Duration Among ALK Fusion Variants in ALK-Positive Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:3383-9. [Crossref] [PubMed]
- Ito K, Hataji O, Kobayashi H, et al. Sequential Therapy with Crizotinib and Alectinib in ALK-Rearranged Non-Small Cell Lung Cancer-A Multicenter Retrospective Study. J Thorac Oncol 2017;12:390-6. [Crossref] [PubMed]
- Sehgal K, Peters MLB, VanderLaan PA, et al. Activity of Brigatinib in the Setting of Alectinib Resistance Mediated by ALK I1171S in ALK-Rearranged Lung Cancer. J Thorac Oncol 2019;14:e1-3. [Crossref] [PubMed]
- Sun TY, Niu X, Chakraborty A, et al. Lengthy progression-free survival and intracranial activity of cabozantinib in patients with crizotinib and ceritinib-resistant ROS1-positive non-small-cell lung cancer. J Thorac Oncol 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Kang J, Chen HJ, Zhang XC, et al. Heterogeneous responses and resistant mechanisms to crizotinib in ALK-positive advanced non-small cell lung cancer. Thorac Cancer 2018;9:1093-103. [Crossref] [PubMed]
- Santarpia M, Gil N, Rosell R. Strategies to overcome resistance to tyrosine kinase inhibitors in non-small-cell lung cancer. Expert Rev Clin Pharmacol 2015;8:461-77. [Crossref] [PubMed]
- Wei J, van der Wekken AJ, Saber A, et al. Mutations in EMT-Related Genes in ALK Positive Crizotinib Resistant Non-Small Cell Lung Cancers. Cancers (Basel) 2018;10:E10. [Crossref] [PubMed]
- Ou SI, Lee TK, Young L, et al. Dual occurrence of ALK G1202R solvent front mutation and small cell lung cancer transformation as resistance mechanisms to second generation ALK inhibitors without prior exposure to crizotinib. Pitfall of solely relying on liquid re-biopsy? Lung Cancer 2017;106:110-4. [Crossref] [PubMed]
- Isozaki H, Takigawa N, Kiura K. Mechanisms of Acquired Resistance to ALK Inhibitors and the Rationale for Treating ALK-positive Lung Cancer. Cancers (Basel) 2015;7:763-83. [Crossref] [PubMed]
- Shaw AT, Friboulet L, Leshchiner I, et al. Resensitization to Crizotinib by the Lorlatinib ALK Resistance Mutation L1198F. N Engl J Med 2016;374:54-61. [Crossref] [PubMed]
- Shiau CJ, Babwah JP, da Cunha Santos G, et al. Sample features associated with success rates in population-based EGFR mutation testing. J Thorac Oncol 2014;9:947-56. [Crossref] [PubMed]
- Cardarella S, Ortiz TM, Joshi VA, et al. The introduction of systematic genomic testing for patients with non-small-cell lung cancer. J Thorac Oncol 2012;7:1767-74. [Crossref] [PubMed]
- Lee JY, Qing X, Xiumin W, et al. Longitudinal monitoring of EGFR mutations in plasma predicts outcomes of NSCLC patients treated with EGFR TKIs: Korean Lung Cancer Consortium (KLCC-12-02). Oncotarget 2016;7:6984-93. [PubMed]
- Mayo-de-las-Casas C, Jordana-Ariza N, Garzón-Ibañez M, et al. Large scale, prospective screening of EGFR mutations in the blood of advanced NSCLC patients to guide treatment decisions. Ann Oncol 2017;28:2248-55. [Crossref] [PubMed]
- Rolfo C, Mack PC, Scagliotti GV, et al. Liquid Biopsy for Advanced Non-Small Cell Lung Cancer (NSCLC): A Statement Paper from the IASLC. J Thorac Oncol 2018;13:1248-68. [Crossref] [PubMed]
- Wang Z, Chen R, Wang S, et al. Quantification and dynamic monitoring of EGFR T790M in plasma cell-free DNA by digital PCR for prognosis of EGFR-TKI treatment in advanced NSCLC. PLoS One 2014;9:e110780. [Crossref] [PubMed]
- Morgillo F, Della Corte CM, Fasano M, et al. Mechanisms of resistance to EGFR-targeted drugs: lung cancer. ESMO Open 2016;1:e000060. [Crossref] [PubMed]
- Jin Y, Shi X, Zhao J, et al. Mechanisms of primary resistance to EGFR targeted therapy in advanced lung adenocarcinomas. Lung Cancer 2018;124:110-6. [Crossref] [PubMed]
- Shan L, Wang Z, Guo L, et al. Concurrence of EGFR amplification and sensitizing mutations indicate a better survival benefit from EGFR-TKI therapy in lung adenocarcinoma patients. Lung Cancer 2015;89:337-42. [Crossref] [PubMed]
- Solomon B, Wilner KD, Shaw AT. Current status of targeted therapy for anaplastic lymphoma kinase-rearranged non-small cell lung cancer. Clin Pharmacol Ther 2014;95:15-23. [Crossref] [PubMed]
- Katayama R. Drug resistance in anaplastic lymphoma kinase-rearranged lung cancer. Cancer Sci 2018;109:572-80. [Crossref] [PubMed]
- Kohsaka S, Nagano M, Ueno T, et al. A method of high-throughput functional evaluation of EGFR gene variants of unknown significance in cancer. Sci Transl Med 2017;9: [Crossref] [PubMed]
- Chic N, Mayo-de-Las-Casas C, Reguart N. Successful Treatment with Gefitinib in Advanced Non-Small Cell Lung Cancer after Acquired Resistance to Osimertinib. J Thorac Oncol 2017;12:e78-80. [Crossref] [PubMed]
- Wang Z, Yang JJ, Huang J, et al. Brief Report: Lung adenocarcinoma harboring EGFR T790M and in trans C797S responds to combination therapy of first and third generation EGFR-TKIs and shifts allelic configuration at resistance. J Thorac Oncol 2017;12:1723-7. [Crossref] [PubMed]
- Burke JE, Perisic O, Masson GR, et al. Oncogenic mutations mimic and enhance dynamic events in the natural activation of phosphoinositide 3-kinase p110alpha (PIK3CA). Proc Natl Acad Sci U S A 2012;109:15259-64. [Crossref] [PubMed]
- Bordi P, Tiseo M, Rofi E, et al. Detection of ALK and KRAS Mutations in Circulating Tumor DNA of Patients With Advanced ALK-Positive NSCLC With Disease Progression During Crizotinib Treatment. Clin Lung Cancer 2017;18:692-7. [Crossref] [PubMed]
- Doebele RC, Pilling AB, Aisner DL, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res 2012;18:1472-82. [Crossref] [PubMed]
- Kato S, Goodman A, Walavalkar V, et al. Hyperprogressors after Immunotherapy: Analysis of Genomic Alterations Associated with Accelerated Growth Rate. Clin Cancer Res 2017;23:4242-50. [Crossref] [PubMed]


