
Effects of ginsenoside Rg3 on apoptosis in A375.S2 melanoma cells
Introduction
The human skin is continuously damaged by various stressors caused by internal or external factors, such as exposure to ultraviolet (UV) rays and free radicals (1,2). Skin exposure to oxidative stress or UV radiation is known to cause aging and tumors (3,4). Meanwhile, the aged population with high UV ray accumulation rates has increased along with the increase in mean life expectancy, and the prevalence of malignant melanoma have therefore risen steadily as well (5,6).
Malignant melanoma comprises 4% of all skin cancer cases, but accounts for 80% of the deaths caused by skin cancer; when it metastasizes, the 10-year survival rate is less than 10% (7,8). Malignant melanoma is difficult to treat through surgery, radiation therapy, or chemotherapy. Despite recent improvements in immunotherapy and target therapy, there is still a great deal of difficulty in treatment due to high cost and toxicity (9,10). As a result, researchers have been trying for decades to find new anti-cancer substances in herbs (11-13).
Ginseng is one of the most commonly used ingredients in traditional medicine, and it is widely used as a medicinal herb for various purposes in many Asian countries (14,15). Numerous studies have found ginseng to have many physiological and pharmacological effects, such as maintaining homeostasis and improving memory. Ginseng has especially reported to have anti-cancer effects for ovarian cancer, breast cancer, lung cancer, and so on (16-20).
Ginsenosides are among the components of ginseng that give ginseng its various pharmacological effects. More than 60 ginsenosides are known, and Rg3 is a ginsenoside that has been reported to have multiple pharmacological and physiological effects (21-23). It has especially been noted to exert anti-cancer effects such as apoptosis induction and anti-proliferative, anti-metastatic, and anti-angiogenetic effects (24-29). Research on malignant melanoma has reported that Rg3 exerted apoptotic and anti-metastatic effects on B16F10 melanoma cells (30,31). However, B16F10 melanoma cells are a cell strain originating from mice, and studies on the anti-cancer effects of Rg3 on melanoma cells originating from humans have been rarely reported. This study, therefore, investigated A375.S2, a melanoma cell line originating from humans that has not been studied previously, and we examined whether the ginsenoside Rg3 induced apoptosis in A375.S2 cells and if so, through which signaling pathways.
The overall structure of this study is as follows: first, this study investigated the cell viability, cell morphology, colony formation ability, and migration of A375.S2 cells after treatment with Rg3. In order to determine whether apoptosis occurred when A375.S2 cells were treated with Rg3, a flow cytometric assay was conducted, western blotting was conducted for apoptotic proteins, and an immunocytochemical analysis was conducted. Finally, the study measured cell viability after treatment with inhibitors of various kinases that are known to be involved in apoptosis to determine which signaling pathway induced apoptosis.
Methods
Reagents and materials
The ginsenoside Rg3 was purchased from NPC & BioTech (Daejon, Korea). The Rg3 was dissolved in dimethyl sulfoxide (DMSO, 5 mg/mL) and stored at −80 °C. Specific kinase inhibitors, PD98059, SB203580, and SP600125, were purchased from Thermo Fisher Scientific (Pittsburgh, PA, USA).
Cell culture
A375.S2 melanoma cells were purchased from the American Type Culture Collection (Manassas, VA, USA). The cells were cultured in minimum essential medium containing 10% fetal bovine serum (FBS), 100 U/mL of penicillin, and 100 µg/mL of streptomycin in an incubator with 5% CO2 at 37 °C.
Effects of Rg3 on A375.S2 cells
Cell viability
A375.S2 cells were treated with Rg3, and a methyl thiazol tetrazolium (MTT) assay was conducted to investigate cell viability. First, the A375.S2 cells were seeded at 1×104 per well into 96 wells, stored for 24 hours at 37 °C in the incubator, and then treated with different Rg3 concentrations (0, 10, 20, 40, 60, and 100 µM). After 24 hours, 10 µL of 5 mg/mL MTT was put into the 96 wells for 3 hours at 37 °C for the MTT assay. The culture medium was completely removed and 100 µL of DMSO was added. Light was blocked out and the wells were put on an orbital shaker for 5 minutes. The absorbance at optical density (OD) 570 nm was measured.
Cell morphology
A375.S2 cells were seeded at 5×104 per well into 12 wells, stored for 24 hours at 37 °C in the incubator, and then treated with different Rg3 concentrations (0, 10, 20, and 40 µM). The different cell shapes at different concentrations were observed using a microscope and photographed.
Colony formation ability
A375.S2 cells were seeded at 1×103 per well into 24 wells and stored for 24 hours. Then, with 5% FBS, they were treated with different Rg3 concentrations (0, 10, 20, and 40 µM). The degree of colony formation was monitored for 5–7 days and a crystal violet assay was conducted. First, 1 mL of 1% paraformaldehyde was added into each well and fixed for 15 minutes at room temperature. Then, the samples were stained with 0.5% crystal violet for 30 minutes and washed with distilled water. After taking a photograph, 200 µL of DMSO was added and dissolved by shaking for 30 minutes. The absorbance at OD 540 nm was measured.
Cell migration ability
A wound healing assay was conducted to investigate A375.S2 cell migration after Rg3 treatment. A375.S2 cells were seeded at 5×105 per well into 6 wells, stored for 24 hours, and cultured into monolayer cells. Then, the center was scratched using a 200 µL pipette tip to make a clean wound area, and washed with Hank’s Balanced Salt Solution (Gibco-Invitrogen, Carlsbad, CA, USA). Then, with 5% FBS, they were treated with 20 µM Rg3, and observed after 24–72 hours using a microscope and photographed. Using ImageJ (National Institutes of Health, Bethesda, MD, USA), the wound distance was measured and quantified.
Confirmation of apoptosis
Flow cytometry assay
Apoptosis was confirmed using an annexin V-FITC/PI apoptosis detection kit (BD Pharmingen, San Diego, CA, USA). A375.S2 cells were seeded at 4×105 per well into 6 wells, stored for 24 hours, and treated with 40 µM Rg3. After 24 hours, the cells were sampled and stained with annexin V and propidium iodide (PI). The sampled cells were resuspended in 1× binding buffer at a concentration of 1×106 cells/mL. Then, 100 µL was removed into a 5-mL tube and mixed with 5 µL of annexin V and 4 µL of PI at room temperature for 15 minutes. Subsequently, 400 µL of 1× binding buffer was added to each tube and was measured using a flow cytometer within 1 hour.
Western blot
A375.S2 cells were seeded at 1×105 per well into 6 wells, stored for 24 hours, and treated with different Rg3 concentrations (0, 5, 10, 20, and 30 µM). After 24 hours, they were sampled and protein was obtained using a lysis buffer. The protein density was measured and equal amounts of protein were loaded onto an acrylamide gel to conduct a western blot test.
Immunocytochemical analysis
Cover slips were put into the well plates and coated with 5 µg/mL of fibronectin for 2 hours at 37 °C. A375.S2 cells were seeded at 5×104 per well into 12 wells, then treated with 20 µM Rg3 the next day. After 24 hours, 2 mL of 4% paraformaldehyde was put into each well and fixed for 15 minutes at room temperature. After they were washed 3 times with phosphate-buffered saline (PBS), they were blocked for a day at 4 °C with 3% bovine serum albumin. Then, the primary antibody was put in for 2 hours to react. After washing 3 times with PBS, the secondary antibody was put in for 2 hours. Lastly, 4',6-diamidino-2-phenylindole (DAPI) was used to stain the samples for 5 minutes, washed using PBS, and observed by a microscope.
Signaling pathway for apoptosis
In order to identify which signaling pathways induced apoptosis, inhibitors of 3 types of kinases known to be involved in apoptosis were prepared: mitogen-activated protein/extracellular signal-related kinase inhibitor (MEK inhibitor: PD98059), p38 mitogen-activated protein kinase inhibitor (p38 MAPK inhibitor: SB203580), and c-Jun N-terminal protein kinase mitogen-activated protein kinase inhibitor (JNK MAPK inhibitor: SP600125).
A375.S2 cells were seeded at 1×104 per well into 96 wells and inhibitors of the 3 types of kinases (50 µM PD98059, 20 µM SB203580, 20 µM SP600125) were put in for 1 hour each, then treated with 20 µM Rg3. After storing for 24 hours in an incubator, MTT assay was conducted.
Statistical analysis
All data were analyzed using SPSS version 24.0 (IBM Corp., Armonk, NY, USA). The MTT assay measurements of cell viability and measurements of colony formation ability were analyzed using the Kruskal-Wallis test, while the measurements for cell migration ability and the MTT assay after using inhibitors were tested using the two-sample t-test. P values <0.05 were considered to indicate statistical significance
Results
A375.S2 cell function changes after Rg3 treatment
Rg3 inhibits A375.S2 cell viability
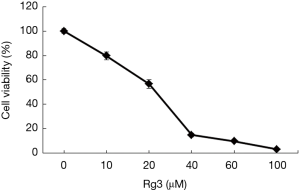
Analyzing the cell viability with an MTT assay after treating A375.S2 cells with different Rg3 concentrations confirmed a significant decrease in cell viability for all Rg3 concentrations (10, 20, 40, 60, and 100 µM) compared to the control groups (P<0.05). The concentration of Rg3 inhibiting the 50% of cell viability (IC50) was 20 µM. An analysis of pairs of groups at successive intervals of Rg3 concentrations showed that groups treated with 40 µM Rg3 and 60 µM Rg3 did not have a significant difference (P>0.05), but all other such pairs showed a significant difference (Figure 1).
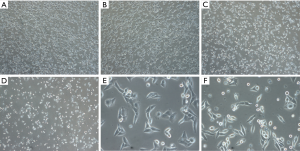
Rg3 changes A375.S2 cell morphology
A375.S2 cells treated with Rg3 changed to have a rounder shape as the Rg3 concentration increased. In addition, the number of cells decreased with Rg3 treatment (Figure 2).
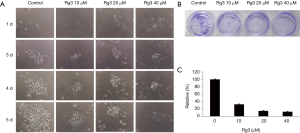
Rg3 inhibits the colony formation ability of A375.S2 cells
When the colony formation ability of A375.S2 cells was analyzed 24 hours after treatment with different Rg3 concentrations, the colony formation ability was found to have decreased for all Rg3 concentration levels compared to the control group (P<0.05). No significant difference was found between the group treated with 20 µM Rg3 and the group treated with 40 µM Rg3 (P>0.05), while the difference between the group treated with 10 µM Rg3 and the group treated with 20 µM Rg3 was significant (P<0.05) (Figure 3).
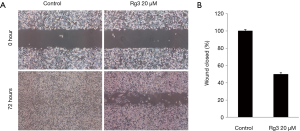
Rg3 inhibits cell migration of A375.S2 cells
When the cell migration ability of A375.S2 cells was tested 72 hours after treatment with 20 µM Rg3, it was found that the cell migration ability decreased in the group treated with Rg3 compared to the control group (P<0.05) (Figure 4).
A375.S2 cell apoptosis after Rg3 treatment
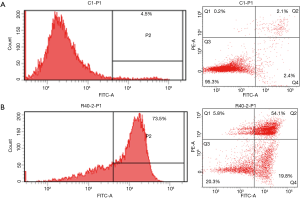
Confirming apoptosis using a flow cytometric assay
When apoptosis was analyzed using a flow cytometric assay 24 hours after A375.S2 cells were treated with 40 µM Rg3, a clear tendency for apoptosis was found in comparison to the control group (Figure 5).
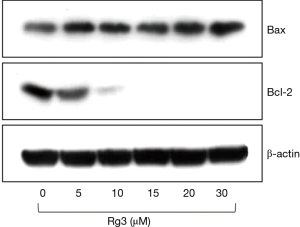
Confirming apoptosis using Western blot
When western blotting was conducted 24 hours after A375.S2 cells were treated with different Rg3 concentrations, it was found that Bax accumulated, while Bcl-2 decreased (Figure 6).
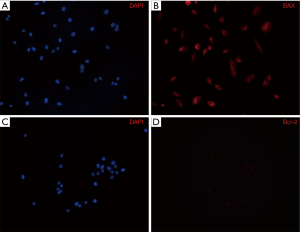
Confirming apoptosis by an immunocytochemical analysis
When apoptosis was analyzed through an immunocytochemical analysis after A375.S2 cells were treated with 20 µM Rg3, the DAPI stain test found that the group treated with Rg3 showed a decrease in cell numbers compared to the control group. Bax accumulated, while the amount of Bcl-2 decreased (Figure 7).
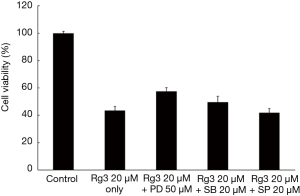
Confirmation of the signaling pathways that induce apoptosis
Involvement of MEK inhibitor in A375.S2 cell apoptosis through Rg3 treatment
The A375.S2 cells were treated with 3 types of inhibitors—MEK inhibitor (PD98059), p38 MAPK inhibitor (SB203580), and JNK MAPK inhibitor (SP600125)—and then treated with 20 µM Rg3. An MTT assay was conducted to check cell viability, and the results showed a significant difference in the group with MEK inhibitor treatment compared to the group only treated with Rg3 (P<0.05). The groups treated with JNK MAPK inhibitor or p38 MAPK inhibitor did not show a significant difference compared to the group treated with only Rg3 (P>0.05) (Figure 8).
Discussion
Rg3 is a ginsenoside found in ginseng that belongs to the class of saponins, and it has been reported to have multiple pharmacological and physiological effects, including anti-canter effects. Zhang et al. reported Rg3 to be effective against lung cancer (32), and Xu et al. reported that Rg3 inhibited the metastasis and growth of cancer in ovarian cancer (17,18). Yuan et al. reported that Rg3 induced apoptosis in colon cancer (19), and Qiu et al. reported that Rg3 induced apoptosis on cancer cells in leukemia (20).
Meanwhile, studies on malignant melanoma have reported that Rg3 exerted apoptotic and anti-metastatic effects on B16F10 melanoma cells (30,31). In a study conducted by Lee et al. in 2014, results of a cell viability test, TUNEL assay, and western blotting indicated that apoptosis occurred in B16F10 melanoma cells when treated with Rg3 (30). Subsequently, Lee et al. reported in their 2015 study, in which they assessed cell viability, cell morphology, cell migration, invasion ability, and colony formation ability, as well as performing a western blot analysis, that the MMP-13 protein was inhibited, confirming that Rg3 inhibited the metastasis of B16F10 cells (31). However, B16F10 melanoma cells are a cell strain originating from mice, meaning that the results of Lee et al. have limitations in terms of clinical applicability. Shan et al. have reported that Rg3 inhibits proliferation of melanoma cells originating in humans (33). But other studies on melanoma cells originating in humans, to determine whether Rg3 has anti-cancer effects, and specifically whether Rg3 can induce apoptosis, have been rarely reported. Furthermore, research the process of Rg3-induced apoptosis and the signaling pathways through which it is induced.
This study investigated the effects of Rg3 on A375.S2 cells, a melanoma cell line originating in humans that has not been studied previously. It examined whether Rg3 induced apoptosis and if so, through which signaling pathways. In order to conduct this research, the author planned a 3-step experiment. First, this study investigated the viability, morphology, colony formation ability, and migration of A375.S2 cells after treatment with Rg3 to see whether any functional changes occurred. An MTT assay found that all groups treated with Rg3 showed decreased cell viability compared to the control group; of particular note, the cell viability decreased by half when treated with Rg3 with a concentration between 20 and 40 µM. Furthermore, the cells changed from a flat form to a rounder shape as the Rg3 concentration increased and cell membrane blebbing occurred. Cell membrane blebbing is known to occur due to F-actin polymerization and actomyosin contractility during the execution phase of apoptosis (34,35). This serves as evidence of the involvement of Rg3 in cell morphology and its ability to induce apoptosis. In addition, a decrease in the number of cells was found, along with morphological changes, as the Rg3 concentration increased. This supports the results presented above regarding cell viability. Colony formation ability and cell migration ability are common characteristics visible in tumor cells, and were also inhibited by Rg3 treatment.
Second, in order to investigate whether apoptosis had taken place, a flow cytometric assay, western blot, and immunocytochemistry analysis were conducted. These 3 experiments all indicated that apoptosis occurred when the A375.S2 cells were treated with Rg3. Apoptosis can generally be divided into the mitochondria-dependent pathway (intrinsic pathway) and the death receptor-dependent pathway (extrinsic pathway) (36-38). The extrinsic cell death pathway is known to be associated with the Fas ligand that induces intracellular signaling and cleavage by attaching to the Fas receptor and activating caspase-8 (39,40). Furthermore, the mitochondria-dependent pathway and death receptor-dependent pathway are both controlled by Bcl-2 family proteins (41,42). The Bcl-2 family contains pro-apoptotic proteins, such as Bax, Bak, Bad, and Bcl-Xs, and anti-apoptotic proteins, such as Bcl-2, Bcl-XL, and Mcl-1 (43). The Bax/Bcl-2 ratio is an important factor in experimental conditions, as it determines whether apoptosis occurs or not (43). In this study, we found that Bax accumulated and Bcl-2 decreased through western blotting and immunocytochemical analysis. This can serve as a critical piece of evidence that Rg3 induces apoptosis.
Finally, the study used an MTT assay to evaluate A375.S2 cells that were treated with Rg3 after the administration of inhibitors of various kinases that are known to be involved in apoptosis. The viability of A375.S2 cells decreased when treated with Rg3 after a MEK inhibitor was administered. This suggests that apoptosis occurring after Rg3 treatment can be induced through the MEK signaling pathway, and serves as additional evidence that A375.S2 cells treated with Rg3 underwent apoptosis.
This study has some limitations in confirming the effect of the inhibitor without confirming the effect of activator treatment in the process of confirming apoptosis signaling pathway, and in that it requires further confirmation of other pathways such as nuclear factor kappa B (NF-κB). Despite these limitations, the results presented above, unlike those of previous studies that did not deal with human-originated A375.S2 melanoma cells, prove that Rg3 can induce apoptosis in human-derived cancer cells. This study is also valuable because it confirmed the relationship between apoptosis and the MEK signaling pathway. Based on these results, future research should analyze whether Rg3 has similar effects on other melanoma cells or has different effects on different types of melanoma cells. Furthermore, research should be conducted on whether Rg3 can treat melanoma cells injected into animals in vivo.
Conclusions
This study confirmed that Rg3, a major active ingredient of ginseng, affected the cell viability, morphology, colony formation ability, and cell migration ability and induced apoptosis in A375.S2 cells, a human-originated melanoma cell strain. Apoptosis was mediated by the MEK signaling pathway. In this study, we found that the ginsenoside Rg3 had an anti-cancer effect on malignant melanoma cells, and we made strides towards better understanding the mechanism. With these results, if clinical studies on ginsenoside Rg3 are continued in the future, it is expected that Rg3 will be applied as an anti-cancer drug for malignant melanoma.
Acknowledgments
Funding: This study was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.11.15). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study is based on the experiments conducted
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wang HM, Chen CY, Chen CY, et al. (-)-N-Formylanonaine from Michelia alba as a human tyrosinase inhibitor and antioxidant. Bioorg Med Chem 2010;18:5241-7. [Crossref] [PubMed]
- Wang HM, Chen CY, Wen ZH. Identifying melanogenesis inhibitors from Cinnamomum subavenium with in vitro and in vivo screening systems by targeting the human tyrosinase. Exp Dermatol 2011;20:242-8. [Crossref] [PubMed]
- Briganti S, Picardo M. Antioxidant activity, lipid peroxidation and skin diseases. What's new. J Eur Acad Dermatol Venereol 2003;17:663-9. [Crossref] [PubMed]
- Picardo M, Grammatico P, Roccella F, et al. Imbalance in the antioxidant pool in melanoma cells and normal melanocytes from patients with melanoma. J Invest Dermatol 1996;107:322-6. [Crossref] [PubMed]
- Lens MB, Dawes M. Global perspectives of contemporary epidemiological trends of cutaneous malignant melanoma. Br J Dermatol 2004;150:179-85. [Crossref] [PubMed]
- Rigel DS, Russak J, Friedman R. The evolution of melanoma diagnosis: 25 years beyond the ABCDs. CA Cancer J Clin 2010;60:301-16. [Crossref] [PubMed]
- Bhatia S, Tykodi SS, Thompson JA. Treatment of metastatic melanoma: an overview. Oncology (Williston Park) 2009;23:488-96. [PubMed]
- Miller AJ, Mihm MC Jr. Melanoma. N Engl J Med 2006;355:51-65. [Crossref] [PubMed]
- Menaa F. Latest approved therapies for metastatic melanoma: what comes next? J Skin Cancer 2013;2013:735282. [Crossref] [PubMed]
- White N, Knight GE, Butler PE, et al. An in vivo model of melanoma: treatment with ATP. Purinergic Signal 2009;5:327-33. [Crossref] [PubMed]
- Srivastava SK, Singh SV. Cell cycle arrest, apoptosis induction and inhibition of nuclear factor kappa B activation in anti-proliferative activity of benzyl isothiocyanate against human pancreatic cancer cells. Carcinogenesis 2004;25:1701-9. [Crossref] [PubMed]
- Ma YS, Hsu SC, Weng SW, et al. Crude extract of Rheum palmatum L induced cell death in LS1034 human colon cancer cells acts through the caspase-dependent and -independent pathways. Environ Toxicol 2014;29:969-80. [Crossref] [PubMed]
- Gao J, Morgan WA, Sanchez-Medina A, et al. The ethanol extract of Scutellaria baicalensis and the active compounds induce cell cycle arrest and apoptosis including upregulation of p53 and Bax in human lung cancer cells. Toxicol Appl Pharmacol 2011;254:221-8. [Crossref] [PubMed]
- Yun SN, Ko SK, Lee KH, et al. Vinegar-processed ginseng radix improves metabolic syndrome induced by a high fat diet in ICR mice. Arch Pharm Res 2007;30:587-95. [Crossref] [PubMed]
- Ang-Lee MK, Moss J, Yuan CS. Herbal medicines and perioperative care. JAMA 2001;286:208-16. [Crossref] [PubMed]
- Koo HN, Jeong HJ, Choi IY, et al. Mountain grown ginseng induces apoptosis in HL-60 cells and its mechanism have little relation with TNF-alpha production. Am J Chin Med 2007;35:169-82. [Crossref] [PubMed]
- Xu TM, Cui MH, Xin Y, et al. Inhibitory effect of ginsenoside Rg3 on ovarian cancer metastasis. Chin Med J (Engl) 2008;121:1394-7. [Crossref] [PubMed]
- Wang JH, Nao JF, Zhang M, et al. 20(s)-ginsenoside Rg3 promotes apoptosis in human ovarian cancer HO-8910 cells through PI3K/Akt and XIAP pathways. Tumour Biol 2014;35:11985-94. [Crossref] [PubMed]
- Yuan HD, Quan HY, Zhang Y, et al. 20(S)-Ginsenoside Rg3-induced apoptosis in HT-29 colon cancer cells is associated with AMPK signaling pathway. Mol Med Rep 2010;3:825-31. [PubMed]
- Qiu XM, Bai X, Jiang HF, et al. 20-(s)-ginsenoside Rg3 induces apoptotic cell death in human leukemic U937 and HL-60 cells through PI3K/Akt pathways. Anticancer Drugs 2014;25:1072-80. [Crossref] [PubMed]
- Jia L, Zhao Y. Current evaluation of the millennium phytomedicine--ginseng (I): etymology, pharmacognosy, phytochemistry, market and regulations. Curr Med Chem 2009;16:2475-84. [Crossref] [PubMed]
- Jia L, Zhao Y, Liang XJ. Current evaluation of the millennium phytomedicine- ginseng (II): Collected chemical entities, modern pharmacology, and clinical applications emanated from traditional Chinese medicine. Curr Med Chem 2009;16:2924-42. [Crossref] [PubMed]
- Wang CZ, Aung HH, Ni M, et al. Red American ginseng: ginsenoside constituents and antiproliferative activities of heat-processed Panax quinquefolius roots. Planta Med 2007;73:669-74. [Crossref] [PubMed]
- Gao JL, Lv GY, He BC, et al. Ginseng saponin metabolite 20(S)-protopanaxadiol inhibits tumor growth by targeting multiple cancer signaling pathways. Oncol Rep 2013;30:292-8. [Crossref] [PubMed]
- Liu TG, Huang Y, Cui DD, et al. Inhibitory effect of ginsenoside Rg3 combined with gemcitabine on angiogenesis and growth of lung cancer in mice. BMC Cancer 2009;9:250. [Crossref] [PubMed]
- Zhang C, Liu L, Yu Y, et al. Antitumor effects of ginsenoside Rg3 on human hepatocellular carcinoma cells. Mol Med Rep 2012;5:1295-8. [PubMed]
- Kim JW, Jung SY, Kwon YH, et al. Ginsenoside Rg3 attenuates tumor angiogenesis via inhibiting bioactivities of endothelial progenitor cells. Cancer Biol Ther 2012;13:504-15. [Crossref] [PubMed]
- Kim YJ, Choi WI, Jeon BN, et al. Stereospecific effects of ginsenoside 20-Rg3 inhibits TGF-beta1-induced epithelial-mesenchymal transition and suppresses lung cancer migration, invasion and anoikis resistance. Toxicology 2014;322:23-33. [Crossref] [PubMed]
- Mochizuki M, Yoo YC, Matsuzawa K, et al. Inhibitory effect of tumor metastasis in mice by saponins, ginsenoside-Rb2, 20(R)- and 20(S)-ginsenoside-Rg3, of red ginseng. Biol Pharm Bull 1995;18:1197-202. [Crossref] [PubMed]
- Lee SG KB, Nam JO. Ginsenoside Rg3 induces Apoptosis in B16F10 Melanoma Cells. Kor J Life Sci 2014;24:1001-5. [Crossref]
- Lee SG, Kang YJ, Nam JO. Anti-Metastasis Effects of Ginsenoside Rg3 in B16F10 Cells. J Microbiol Biotechnol 2015;25:1997-2006. [Crossref] [PubMed]
- Zhang Q, Kang X, Zhao W. Antiangiogenic effect of low-dose cyclophosphamide combined with ginsenoside Rg3 on Lewis lung carcinoma. Biochem Biophys Res Commun 2006;342:824-8. [Crossref] [PubMed]
- Shan X, Aziz F, Tian LL, et al. Ginsenoside Rg3-induced EGFR/MAPK pathway deactivation inhibits melanoma cell proliferation by decreasing FUT4/LeY expression. Int J Oncol 2015;46:1667-76. [Crossref] [PubMed]
- Coleman ML, Sahai EA, Yeo M, et al. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol 2001;3:339-45. [Crossref] [PubMed]
- Sebbagh M, Renvoize C, Hamelin J, et al. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat Cell Biol 2001;3:346-52. [Crossref] [PubMed]
- Thornberry NA, Rano TA, Peterson EP, et al. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J Biol Chem 1997;272:17907-11. [Crossref] [PubMed]
- Lai E, Teodoro T, Volchuk A. Endoplasmic reticulum stress: signaling the unfolded protein response. Physiology (Bethesda) 2007;22:193-201. [Crossref] [PubMed]
- Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev 2007;87:99-163. [Crossref] [PubMed]
- Ashkenazi A, Dixit VM. Apoptosis control by death and decoy receptors. Curr Opin Cell Biol 1999;11:255-60. [Crossref] [PubMed]
- Kamachi M, Le TM, Kim SJ, et al. Human autoimmune sera as molecular probes for the identification of an autoantigen kinase signaling pathway. J Exp Med 2002;196:1213-25. [Crossref] [PubMed]
- Lavrik IN, Golks A, Krammer PH. Caspases: pharmacological manipulation of cell death. J Clin Invest 2005;115:2665-72. [Crossref] [PubMed]
- Orrenius S. Reactive oxygen species in mitochondria-mediated cell death. Drug Metab Rev 2007;39:443-55. [Crossref] [PubMed]
- Merry DE, Korsmeyer SJ. Bcl-2 gene family in the nervous system. Annu Rev Neurosci 1997;20:245-67. [Crossref] [PubMed]


