
Gastric yolk sac tumor with synchronous liver metastasis: first case report in China
Introduction
Yolk sac tumors (YSTs) are uncommon germ cell tumours which were firstly described in gonads, but approximately 5% arise in extragonadal localization, mainly in mid-line locations such as the mediastinum, retroperitoneum, pineal gland and sacrococcygeal region (1,2). YSTs, however, rarely occur in the stomach and only fifteen cases have been reported previously in English literature (3,4). Gastric YSTs are often misdiagnosed as gastric cancer because they are rare and have no typical clinical symptoms (3,5). Patients with gastric YST have a poor prognosis and often have widespread metastasis at the time of diagnosis, and the most common sites of metastasis are the retroperitoneum, lung, liver and bones (3,6). Five cases with gastric YSTs had rapidly fatal clinical courses and died within six weeks, consistent with the highly aggressive nature of these neoplasms at other sites (6). Therefore, it is necessary to have a more comprehensive understanding of the clinical characteristics of this disease so as to achieve early diagnosis and treatment. Here, we report a case of gastric YST without relevant clinical symptoms, only liver mass as the first performance. To the best of our knowledge, the gastric YST presented in this case is the first reported in China. We believe this case will contribute to understanding the clinical characteristics of this rare disease.
Case presentation
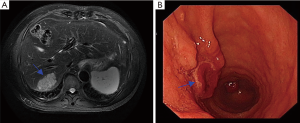
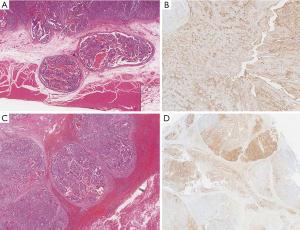
A 63-year-old man was referred to our hospital with a presumed diagnosis of liver mass, which was found incidentally during a routine ultrasonography examination. He did not smoke, drink alcohol, or use intravenous drugs. His medical history was unremarkable with the exception of mild hypertension. Serum alpha-fetoprotein (AFP) levels were elevated to 698.8 ng/mL (normal range, 0–25 ng/mL), whereas serum carcinoembryonic antigen (CEA) and cancer antigen 19-9 (CA 19-9) levels were within the normal limits. His liver function tests were normal, and he was negative for hepatitis B antigen and hepatitis C antibodies. Computed tomography (CT) of the abdomen showed no apparent abnormality except a potentially malignant mass in liver segment VII. Liver magnetic resonance imaging (MRI) showed an isolated heterogeneous enhanced tumor in liver segment VII (Figure 1A). Endoscopic examination showed a Borrmann type I tumor with a reddish surface (1.5×1.5 cm) in the antrum of the stomach (Figure 1B). A biopsy of the tumor suggested adenocarcinoma with moderately poor differentiation. Gastric carcinoma with synchronous liver metastasis was considered as the initial diagnosis. After signing the informed consent and exclusion of contraindications, the patient underwent simultaneous resection of the primary gastric lesion and liver metastasis. The final diagnosis depended on the postoperative pathological examination with technical assistance from the UCLA Medical Center Clinical and Pathology laboratories. Yolk sac components were noted in both the stomach and liver, characterized by reticular, glandular, microcystic, solid growth patterns and Schiller-Duval bodies (Figure 2A,B). Immunohistochemically, the YST components were positive for low-molecular-weight cytokeratins (8, 18, and 19), SALL4, and AFP (Figure 2C,D). Adenocarcinoma components were present only in the stomach. Patient was briefed about the poor outcome of the disease and given choice to chemotherapy for adjuvant therapy, for which he declined. So, the patient did not receive further treatment because of no other effective treatment available. Patient was followed up once every 2–3 months, and the examination included serum AFP level, chest CT, and enhanced CT of the whole abdomen. A recent examination and imaging evaluation showed no sign of recurrence. But the Serum AFP level was elevated to 100.8 ng/mL from the normal level after surgery. The patient’s disease-free survival is over 12 months thus far.
Discussion
Primary hepatic malignancies include hepatocellular carcinoma and cholangiocarcinoma; the former is often accompanied by a history of hepatitis and an elevated AFP level, while the latter is often associated with increased CA 19-9 and bilirubin levels. Our subject had an elevated AFP level only, which is not completely consistent with the diagnosis of primary hepatic malignancies. Metastatic hepatic carcinomas are not rare, with gastrointestinal tumor metastasis to the liver being the most common. Certainly, the most direct way to determine the nature of a tumor is needle biopsy. However, due to the low volume of needle biopsy tissue, pathological diagnosis is difficult and can even result in a missed diagnosis. Therefore, we performed gastrointestinal endoscopy as the next step to exclude the possibility of gastrointestinal tumor metastasis. In our present case, the gastroscopy examination showed a mass in the gastric antrum, but the patient was misdiagnosed with poorly differentiated adenocarcinoma by needle biopsy. The diagnosis was confirmed postoperatively as gastric adenocarcinoma with yolk sac differentiation with concomitant hepatic metastasis (YST only).
Gastric YSTs is a rare entity with only 15 cases being reported in English literature from 1985, and 10 cases (66.7%) being reported to have adenocarcinoma components as well (Table 1). The pathogenesis of gastric YSTs has not been determined. One hypothesis is that retrodifferentiation of adenocarcinoma may result in the development of gastric YSTs, due to most of the gastric YSTs being accompanied by adenocarcinomatous components (7,8). This hypothesis was supported by genotyping analysis that showed the same pattern of p53 mutation in the adenocarcinomatous and yolk sac components (8). Another hypothesis is that, similar to other gonadal germ cell tumors, gastric YSTs may arise from migrating germ cells during embryonal development (9). Because adenocarcinoma components were observed in our case, the gastric YST of our patient may have originated from primary gastric adenocarcinoma.
Table 1
| Authors (year) | Age/gender | AFP (ng/mL) | Metastases | Therapy | Histology | Prognosis |
|---|---|---|---|---|---|---|
| Garcia et al. (1985) (7) | 65/M | NM | Liver | NT | YST, CC, AC | Died in 40 days |
| Motoyama et al. (1985) (8) | 72/F | 51.0 | None | S | YST, AC | Survival for 3 years |
| Zámecník et al. (1993) (9) | 88/M | NM | LN, retroperitoneum, omentum | S | YST | Died in 4 weeks |
| Suzuki et al. (1999) (10) | 56/M | 1,768 | LN, peritoneum | S, CT | YST, AC | Died in 6 months |
| Puglisi et al. (1999) (11) | 61/M | 1,050 | Abdominal cavity | S | YST, AC | Died in 1 month |
| Wang et al. (2000) (12) | 36/M | 38,200 | LN, lung, mediastinum | CT | YST, AC | Lost to follow-up after 1 month |
| Napaki et al. (2004) (13) | 38/F | 231 | liver | S | YST, AC | Survival for 32 months |
| Kanai et al. (2005) (14) | 87/M | High | None | S | YST | Died in 7 months |
| Tahara et al. (2008) (15) | 74/M | 523 | LN, liver, lung | NT | YST | Died in 6 days |
| Kim et al. (2009) (3) | 61/M | 50 | None | S | YST | No recurrence for 3 months |
| Magni et al. (2010) (5) | 62/M | NM | None | S, CT | YST | Died in 12 months |
| Satake et al. (2011) (16) | 74/M | 35.7 | LN, liver | S, RF | YST, CC, AC | Recurrence in 8 months |
| Bihari et al. (2013) (6) | 50/M | 2,291 | Liver | NT | YST, AC | No follow-up |
| Yalaza et al. (2017) (4) | 68/F | 50 | LN | S, CT | YST, AC | Died in 8 months |
| Ibrahim et al. (2018) (17) | 86/F | 6,590 | None | S | YST | No follow-up |
| Present case | 63/M | 698.8 | Liver | S | YST, AC | Survival for 12 months |
YST, yolk sac tumor; AFP, alpha-fetoprotein; AC, adenocarcinoma; CC, choriocarcinoma; LN, lymph node; NM, not mentioned; NT, no treatment; S, surgery; CT, chemotherapy; RFA, radiofrequency ablation.
Gastric YSTs generally affect middle aged and elderly people with male preponderance, at a mean age of 65.2 years, but the clinical symptoms are often nonspecific and include epigastric pain, hematemesis, anorexia, abdominal fullness, and weight loss (3). Our patient showed no clinical symptoms, but a liver mass was found incidentally during a routine physical examination. Histologically, a YST exhibits a reticular pattern (a clear-cell endoblastic pattern) with glomerulus-like structures (Schiller-Duval bodies) (10). Also, the serum AFP level in most cases is usually high (Table 1), as a result of high YST cell production, and AFP can be detected immunohistochemically within the tumor (11). The postoperative pathology of our case was consistent with the above manifestations. After tumor excision, AFP levels in patients often drop rapidly (3,5,12-16). According to reported literature, 5 cases developed systemic relapse accompanied by an increase in AFP (5,7,13-15). Although the reported number of YSTs is not so large to predict a proper outcome, the serum levels of AFP may be a prognostic marker for recurrence and survival of gastric YST (10,17). The serum AFP level in our case decreased to nearly within the normal range after surgery but has gradually increased recently which may indicate poor prognosis. The prognosis of gastric YSTs is generally poor, most cases dying within 1 year after diagnosis, because many patients already have widespread metastases by the time of diagnosis (18). Adjuvant chemotherapy agents, such as cisplatin, vinblastine, bleomycin and etoposide, have not been shown to improve long-term survival of patients with gastric YSTs (5,7,18). Despite the presence of germ cell components, they seem to have poor responses to chemotherapy. At present, there is no large-scale clinical research about YSTs-related targeted therapy. A recent study showed that the programmed cell death 1 (PD-1)/programmed death ligand 1 (PD-L1) pathway could be a novel therapeutic target in testicular germ cell tumors (19). However, the effect on yolk cystic tumor is still unknown. The effective therapeutic approach beyond early diagnosis and curative resection is still uncertain. Gastric YSTs treatment still needs further study and clinical data accumulation in order to confirm which treatment is the most effective.
Conclusions
For the first time we report here on a case of gastric YST presenting liver mass as the first performance and the patient was cured after simultaneous resection of the primary gastric lesion and liver metastasis in China. In conclusion, in patients with gastric tumor with a high level of AFP in the serum in absence of gonadal tumor and hepatocellular carcinoma, gastric YST is the likely diagnosis. AFP may be a prognostic marker for recurrence and survival of gastric YST. Histologically, a reticular pattern (a clear-cell endoblastic pattern) with glomerulus-like structures (Schiller-Duval bodies) is golden criteria of diagnosis for YST.
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.01.04). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Consent was obtained from relatives of the patient for publication of this report and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Toner GC, Geller NL, Lin SY, et al. Extragonadal and poor risk nonseminomatous germ cell tumors. Survival and prognostic features. Cancer 1991;67:2049-57. [Crossref] [PubMed]
- Shayegan B, Carver BS, Stasi J, et al. Clinical outcome following post-chemotherapy retroperitoneal lymph node dissection in men with intermediate- and poor-risk nonseminomatous germ cell tumour. BJU Int 2007;99:993-7. [Crossref] [PubMed]
- Kim YS, Kim SH, Seong JK, et al. Gastric yolk sac tumor: a case report and review of the literature. Korean J Intern Med 2009;24:143-6. [Crossref] [PubMed]
- Yalaza M, Kafadar MT, Turkan A. Gastric cancer with adenocarcinoma and yolk sac tumor components: A rare entity. North Clin Istanb 2017;4:275-8. [PubMed]
- Magni E, Sonzogni A, Zampino MG. Primary pure gastric yolk sac tumor. Rare Tumors 2010;2:e10. [Crossref] [PubMed]
- Bihari C, Rastogi A, Chandan KN, et al. Gastric adenocarcinoma with yolk sac tumor differentiation and liver metastasis of yolk sac tumor component. Case Rep Oncol Med 2013;2013:923596. [Crossref] [PubMed]
- Wang L, Tabbarah HJ, Gulati P, et al. Gastric adenocarcinoma with a yolk sac component: a case report and review of the literature. J Clin Gastroenterol 2000;31:85-8. [Crossref] [PubMed]
- Puglisi F, Damante G, Pizzolitto S, et al. Combined yolk sac tumor and adenocarcinoma in a gastric stump: molecular evidence of clonality. Cancer 1999;85:1910-6. [Crossref] [PubMed]
- Wurzel J, Brooks JJ. Primary gastric choriocarcinoma: immunohistochemistry, postmortem documentation, and hormonal effects in a postmenopausal female. Cancer 1981;48:2756-61. [Crossref] [PubMed]
- Garcia RL, Ghali VS. Gastric choriocarcinoma and yolk sac tumor in a man: observations about its possible origin. Hum Pathol 1985;16:955-8. [Crossref] [PubMed]
- McKenney JK, Heerema-McKenney A, Rouse RV. Extragonadal germ cell tumors: a review with emphasis on pathologic features, clinical prognostic variables, and differential diagnostic considerations. Adv Anat Pathol 2007;14:69-92. [Crossref] [PubMed]
- Satake N, Chikakiyo M, Yagi T, et al. Gastric cancer with choriocarcinoma and yolk sac tumor components: case report. Pathol Int 2011;61:156-60. [Crossref] [PubMed]
- Kanai M, Torii A, Hamada A, et al. Pure gastric yolk sac tumor that was diagnosed after curative resection: case report and review of literature. Int J Gastrointest Cancer 2005;35:77-81. [Crossref] [PubMed]
- Napaki S. Combined yolk sac tumour and adenocarcinoma of the oesophago-gastric junction. Pathology 2004;36:589-92. [Crossref] [PubMed]
- Suzuki T, Kimura N, Shizawa S, et al. Yolk sac tumor of the stomach with an adenocarcinomatous component: a case report with immunohistochemical analysis. Pathol Int 1999;49:557-62. [Crossref] [PubMed]
- Motoyama T, Saito K, Iwafuchi M, et al. Endodermal sinus tumor of the stomach. Acta Pathol Jpn 1985;35:497-505. [PubMed]
- Ibrahim A, MacDermid E, Nguyen HPT, et al. Massive pure gastric yolk sac tumour: a unique presentation of a rare pathology. ANZ J Surg 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Ukiyama E, Endo M, Yoshida F, et al. Recurrent yolk sac tumor following resection of a neonatal immature gastric teratoma. Pediatr Surg Int 2005;21:585-8. [Crossref] [PubMed]
- Cierna Z, Mego M, Miskovska V, et al. Prognostic value of programmed-death-1 receptor (PD-1) and its ligand 1 (PD-L1) in testicular germ cell tumors. Ann Oncol 2016;27:300-5. [Crossref] [PubMed]


