
Clinical application of combined laparoscopic surgery in the treatment of primary hepatocellular carcinoma with portal hypertension: a report of 16 cases
Introduction
There are >200 million Chinese patients chronically infected with hepatitis B virus, which often results in liver cirrhosis and portal hypertension (PHT) (1,2). In total, 70–85% of hepatocellular carcinoma (HCC) results from compensatory hypertrophy of hepatocytes in hepatitis and hepatocirrhosis (3). Therefore, primary HCC is a common malignant tumor in China, which is often accompanied by PHT (4). To date, liver transplantation is considered the best treatment for early-stage HCC with clinical manifestations on decompensated liver cirrhosis, such as PHT. However, only few patients undergo liver transplantation due to the insufficient number of donors and high cost of surgeries, which complicate treatments (5). Simple excision of liver lesions may remarkably increase the risk of operation when the patient has gastroesophageal varices and hypersplenism caused by PHT, accompanied by severe liver cirrhosis, poor liver function and co-existing clotting disorders. In a previous study (6), partial hepatectomy temporarily raised portal venous pressure, which might cause variceal bleeding and even mortality in patients with gastroesophageal varices and hypersplenism. Whole or partial splenic embolization has been proposed as a method to control hypersplenism, which is an important contraindication of surgery. Considering the various complications associated, such as splenic abscess, and the inadequate treatment for hypersplenism, embolization is not recommended by experts. Furthermore, in the two-staged therapy, i.e., splenic devascularization or embolization followed by hepatectomy, patients suffer surgical strikes twice and miss the best operation time for prolonged treatment, since the tumor grows and spreads fast. Whether simultaneous operation can be used in patients with liver cirrhosis and PHT accompanied by HCC is controversial (7-10). Simultaneous laparotomy for HCC and PHT, as well as laparoscopic hand-assisted hepatectomy and splenectomy, have been reported. However, previous studies on laparoscopy were limited to hepatectomy combined with splenectomy and pericardial devascularization.
Combined operation of hepatectomy with splenectomy and pericardial devascularization has been reported as an effective treatment. However, two surgical sets located in the upper left or right abdomen increase the size of the operative incision, which can affect patients’ homeostasis and immunity and have adverse effects on postoperative recovery. Furthermore, radiofrequency ablation (RFA) can be performed initially in certain patients with HCC, and splenectomy and portal-azygous disconnection are then received in the second stage of operation. RFA is not a radical therapy despite its clinical value in these two co-existing diseases, and is only suitable for patients with small-size HCC (≤3 cm). However, RFA is unable to inactivate tumor cells completely when the tumor size is >3 cm. In addition, tumors on the liver surface or edges and nearby organs cannot be removed via RFA. Recently, simultaneous laparoscopic hand-assisted hepatectomy, splenectomy and portal-azygous disconnection has been reported as a therapy for non-Chinese patients with HCC and PHT (4). The combined surgery not only treats HCC and PHT at the same time, but also avoids the remarkable postoperative wound. With the development and popularization of minimally invasive techniques in hepatobiliary surgery, the technique of laparoscopic partial/half hepatectomy, splenectomy and portal-azygous disconnection has been gradually improved. In our department, its clinical practice has been applied since 2011. The combined operation has been performed to date in a total of 16 patients, all of whom recovered well. The present study reports the details of laparoscopic hepatectomy, splenectomy and portal-azygous disconnection.
Methods
Clinical characteristics of the patients
In total, 16 patients were treated with laparoscopic surgery for PHT and HCC from April 2011 (Table 1). The present study was approved by the Ethics Review Board at The Third Military Medical University (2011KYNO.56), and written informed consent was obtained from all patients prior to participation. All patients were diagnosed with HCC by abdominal contrast-enhanced dynamic computed tomography (CT) and/or enhanced magnetic resonance imaging (MRI) in the preoperative examination, and were confirmed to have HCC by pathological examination following surgery. All patients had been diagnosed with PHT, defined as the presence of esophageal varices and/or a platelet count of <100,000 cells/µL in association with splenomegaly. Presence of esophageal varices was determined preoperatively based on upper gastrointestinal endoscopic findings. Splenomegaly was diagnosed as present when the spleen length exceeded 10 cm on preoperative CT and/or MRI. Chronic hepatitis B and posthepatitic cirrhosis in different degrees were observed in cancer-free liver tissue. A total of 13 patients visited their doctors for recurrent hemorrhage in the digestive tract (hematemesis and/or melena). Middle and severe varicose veins in the lower esophagus were all diagnosed in preoperative examination. In preoperative assessment, the liver function was classified as A–B Child-Pugh score; focal liver lesions were observed in imaging examinations (tumor size >3 cm), which were located in a different segment on the liver surface; different degrees of splenic enlargement, hypersplenism and pancytopenia were observed. Of the patients, 5 were negative and 11 were positive (who became negative following operation) in alpha fetal protein (AFP). In total, 6 patients received adefovir and lamivudine for antiviral treatment for >1 year prior to surgery, while 10 had not received prior antiviral treatment but received individual antiviral treatment during hospitalization. Long-term individualized antiviral treatment was continued in all patients upon surgery. All patients were followed up at the outpatient clinic. The imaging follow-up examinations included MRI or CT at 3 months, and then MRI every 6 months. The follow-up time was 3 years.
Table 1
| Indicators | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | Patient 10 | Patient 11 | Patient 12 | Patient 13 | Patient 14 | Patient 15 | Patient 16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Male | Female | Male | Male | Male | Female | Female | Male | Male | Female | Male | Female | Female | Male | Female | Male |
| Age (years) | 45 | 63 | 54 | 31 | 57 | 49 | 55 | 56 | 67 | 62 | 58 | 29 | 70 | 58 | 62 | 45 |
| Child-Pugh Class | A | A | B | A | A | A | B | A | A | B | A | A | A | B | A | A |
| HCC | ||||||||||||||||
| Liver segment | IVa | VI | VIII | V | VI | VIII | V | VI | IVb | VI | VII | V | VIII | IVa | V | VI |
| Tumor diameter (cm) | 3.5 | 3.8 | 4.8 | 2.5 | 3 | 4.5 | 5 | 2 | 5 | 3.5 | 1 | 3 | 2 | 1.5 | 2 | 1.5 |
| Upper gastrointestinal hemorrhage | ||||||||||||||||
| Hematemesis (times) | 2 | 1 | 0 | 0 | 0 | 3 | 0 | 2 | 4 | 1 | 1 | 0 | 0 | 1 | 2 | 1 |
| Melena (times) | 0 | 2 | 3 | 0 | 0 | 0 | 2 | 1 | 0 | 2 | 0 | 3 | 0 | 2 | 0 | 1 |
| Operation date | 2011/5/3 | 2011/4/21 | 2011/11/16 | 2012/9/7 | 2013/5/10 | 2014/3/14 | 2013/8/6 | 2013/10/24 | 2013/4/9 | 2013/3/28 | 2013/8/1 | 2015/5/11 | 2014/12/17 | 2014/12/9 | 2014/8/29 | 2014/6/3 |
| ALT (IU/L) | 26 | 27 | 16 | 37 | 32 | 41 | 64 | 44 | 28 | 48 | 29 | 15 | 23 | 44 | 72 | 35 |
| AST (IU/L) | 31 | 42 | 35 | 51 | 34 | 45 | 47 | 37 | 47 | 54 | 38 | 34 | 31 | 52 | 84 | 55 |
| Albumin (g/L) | 44 | 37.4 | 31.9 | 35.6 | 36.3 | 35.1 | 39.7 | 32.5 | 42 | 31.8 | 35.4 | 37.6 | 46.3 | 37 | 33.9 | 30.6 |
| Hemoglobin (g/L) | 114 | 94 | 81 | 103 | 112 | 108 | 98 | 109 | 95 | 112 | 89 | 108 | 102 | 93 | 89 | 105 |
| White blood cells (109 cells/L) | 2.97 | 1.27 | 2.17 | 1.71 | 2.16 | 1.93 | 1.85 | 2.3 | 1.94 | 2.11 | 1.76 | 1.57 | 1.87 | 2.03 | 1.92 | 2.4 |
| Blood platelets (109 cells/L) | 29 | 23 | 16 | 21 | 35 | 19 | 24 | 28 | 21 | 26 | 22 | 19 | 20 | 27 | 23 | 26 |
| AFP (ng/mL) | 19 | 379 | 481 | >1,210 | 498 | >1,210 | 9 | 59 | 993 | 36.5 | 485 | >1,210 | 4.5 | 20 | 192 | 3 |
| ICGR15 (%) | 9.8 | 21 | 40 | 52 | 46 | 63 | 8.3 | 18 | 53 | 36 | 42 | 50 | 12 | 15 | 28 | 16 |
| HBV-DNA (copy/mL) | 130 | 500 | 422 | 2,500 | <500 | 3,600 | 260 | 313 | 185 | 406 | 341 | 168 | 220 | 430 | 279 | 194 |
HCC, hepatocellular carcinoma; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AFP, alpha fetal protein; ICGR15, indocyanine green retention rate at 15 min.
Surgical procedure
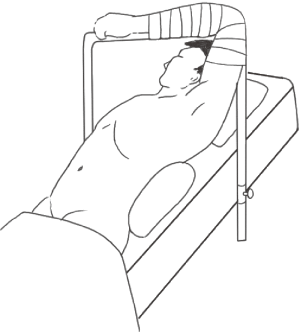
Laparoscopic splenectomy, pericardial devascularization and partial hepatectomy were performed simultaneously. Patients were in supine position, with endotracheal intubation and under general anesthesia (Figure 1). An incision was performed 1 cm under the umbilicus. Then, CO2 pneumoperitoneum was established and a 10-mm peritoneoscope was placed in the trocar hole. Additional trocar holes were performed, as depicted in Figure 2. Abdominal pressure ranged from 12 to 14 mmHg. During surgery, patient’s position was adjusted according to the parts of splenectomy and hepatectomy required for exposure of surgical field and easy operation. Splenectomy was performed first, followed by pericardial devascularization and hepatectomy. This method was able to increase the amount of blood platelets and improve coagulation function following splenectomy while decreasing the risk of hemorrhage during and subsequent to hepatectomy.
Megalosplenic resection
The left side of the body was positioned higher than the right by 30º, and the head was positioned higher than the feet by 15º. First, a gastrocolic ligament was cut by an ultrasonic knife. The splenic artery was dissociated and ligated on the superior border of pancreas to shrink the spleen and decrease tension, which benefited the dissociation and excision of the spleen. Second, the vasa brevia was cut upward along with greater curvature to dissociate the upper limit of the spleen. Third, the spleen and colon ligament were dissected and dissociated to free the lower limit of the spleen. Next, the lienorenal ligament and splenic and pancreatic ligament were broken once the spleen was turned towards the upper right in order to uncover the back of the splenic hilus. Lastly, the splenic hilus was detached through primary splenic pedicle transaction with a 4.5-cm Endo-Linear stapler. Then, the lienophrenic ligament was cut and the spleen was detached once the spleen was lifted to the upper right.
Pericardial devascularization
An assistant pulled the stomach to the lower left to show lesser curvature. The stomach, esophagus and upper esophagus branches were searched in the upper coronary artery along with lesser curvature. Variceal veins in the fundus of the stomach and left esophagus, including the left inferior phrenic vein, were dissected by ultrasonic knife. Wider blood vessels were clipped by biological or Hem-O-lok clamps first. Then, the assistant pulled the stomach to the upper right. The posterior gastric veins and variceal veins on the back of the esophagus were reduced to 8 cm in the lower esophagus.
Partial hepatectomy
The patient’s position was changed, and the right side of the body was located higher than the left side by 30º, while the head was positioned higher than the feet by 15º. The tumor was positioned and no sub or metastasis focus was identified in ultrasonography during the operation. Then, the liver parenchyma was detached via ultrasonic knife. The margin of unregular hepatectomy was >1 cm apart from the tumor border. The mean blood loss was 350 mL during the operation. Since these patients had severe liver cirrhosis and poor liver reserve function, a large area hepatectomy was unsuitable; thus, only partial hepatectomy on the liver surface or edge could be performed. Moreover, the serious liver cirrhosis made the liver less tolerable to ischemia reperfusion, and increased vascular resistance in the portal vein, which might lead to bleeding in wounds around the splenic hilum, esophagus and cardia. Thus, the first porta hepatis was not blocked during the operation.
Postoperative treatment
Ascites appeared in 16 patients under conventional liver-protecting and antiviral treatments. Thus, blood plasma and/or serum albumin and diuretics were used to maintain urine output at ≥2,000 mL/day and keep the balance of water and electrolytes. An intravenous drip of low-molecular weight dextran was administered for prophylactic anticoagulation at postoperative day (POD) 1. Oral aspirin (100 mg/day) was administered to prevent blood clotting and thrombus at POD 3, when patients were able to eat. Blood examinations were performed regularly. Subcutaneous injections of low-molecular weight heparin calcium (4,100 IU) were used for anticoagulation therapy when the number of blood platelets was >500×109 cells/L upon operation. Abdominal ultrasonography was used at POD 7 to check portal vein flow and thrombosis. Platelet filtration treatment was necessary in patients with blood platelets >1,000×109 cells/L.
Statistical analysis
Statistical analysis was performed with SPSS 21.0. All quantitative data are expressed as the mean ± standard deviation and all qualitative data are expressed as n (%).
Results
The operative and postoperative data are summarized in Table 2. The operation time and volume of blood loss were 336±18 min and 337±351 mL, respectively. In total, 2 patients received intraoperative homologous blood transfusion and 9 patients received plasma transfusion. The time for diet intake, drainage time and duration of hospital stay were 3.5±0.5), 7.3±1.0 and 13.6±3.6 days, respectively. The postoperative complications included 1 patient with anastomosis-site bleeding, 2 patients with abdominal effusion and 3 patients with portal vein thrombosis, which were treated conservatively. The overall survival rates at years 1 and 3 were 100% (16/16) and 87.5% (14/16), respectively. The recurrence-free survival rates at years 1 and 3 were 87.5% (14/16) and 62.5% (10/16), respectively.
Table 2
| Indicators | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | Patient 10 | Patient 11 | Patient 12 | Patient 13 | Patient 14 | Patient 15 | Patient 16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| During operation | ||||||||||||||||
| Duration (min) | 336 | 354 | 306 | 356 | 342 | 339 | 360 | 325 | 309 | 312 | 328 | 341 | 352 | 324 | 334 | 362 |
| Blood loss (mL) | 150 | 1,500 | 200 | 180 | 230 | 350 | 400 | 200 | 200 | 300 | 150 | 300 | 150 | 200 | 800 | 80 |
| RBC transfusion (mL) | 0 | 1,200 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 600 | 0 |
| Plasma transfusion (mL) | 0 | 740 | 0 | 500 | 450 | 500 | 500 | 0 | 0 | 500 | 0 | 500 | 0 | 400 | 500 | 0 |
| Spleen size (cm3) | 1,936 | 1,462.50 | 1,840 | 2,079 | 1,400 | 1,540 | 2,024 | 1,680 | 1,748 | 1,955 | 1,760 | 2,280 | 1,881 | 2,376 | 2,475 | 1,260 |
| Tumor diameter (cm) | 2 | 2.5 | 4.8 | 2 | 2.5 | 4.8 | 3 | 2.9 | 3.6 | 2.5 | 3.6 | 4 | 3.8 | 3.2 | 4.4 | 3.2 |
| Resection range (cm)a | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Postoperation | ||||||||||||||||
| Oral intake (days) | 3 | 4 | 3 | 4 | 4 | 3 | 3 | 4 | 4 | 4 | 3 | 4 | 3 | 3 | 4 | 3 |
| Drainage time (days) | 6 | 9 | 7 | 6 | 7 | 8 | 8 | 7 | 7 | 9 | 6 | 6 | 7 | 8 | 7 | 8 |
| Hospital stay (days) | 9 | 24 | 11 | 10 | 12 | 13 | 14 | 12 | 14 | 19 | 12 | 13 | 14 | 14 | 11 | 15 |
a, distance from tumor margin. RBC, red blood cell.
Patients could get out of bed in the early period of postoperation and recovered quickly from the minimal invasive surgical therapy through laparoscopy. The average time of postoperative hospitalization was 13.6 days. Hepatic lesions were diagnosed as HCC in postoperative pathologic examinations. The first porta hepatis was not blocked during the operation; thus, all the indicators on liver function were mildly increased. Through antiviral and liver-protecting treatments, all patients regained nearly normal liver function. Surgical order, splenectomy, pericardial devascularization and partial hepatectomy raised the number of platelets, improved the function of blood coagulation, and decreased the risk of hemorrhage during and subsequent to surgery. In pericardial devascularization, laparoscopy had more merits than routine laparotomy in enlarging surgical field and revealing high esophageal veins. Despite rigorous monitoring of blood examinations, particularly changes in blood platelet number, and early applications of anticoagulants subsequently to the operation, portal vein thrombosis at different levels was still observed in 3 patients. By strengthening the anticoagulation, controlling the rise in blood platelets and monitoring portal vein flow, the thrombosis, as mural thrombus, did not cause obstruction in the portal vein or aggravated PHT. No apparent changes were evidenced in liver function tests.
Discussion
Hanazaki et al. (11) reported that simultaneous operation in patients with HCC and PHT could increase the risk of portal vein thrombosis, resulting in liver failure and mortality. However, staging surgery would extend the time of tumor growth, and patients may miss their best therapy for the sustained growth and metastasis of tumor. Furthermore, reducing liver and lesions was not avoidable during operation, which could increase the risk of tumor diffusion and metastasis. Consequently, hardeners were suggested to be injected repeatedly for the treatment of esophageal varices following liver cancer resection (12,13). Sugawara et al. (14) reported that all 48 patients recovered uneventfully following simultaneous or staging operations. The two groups had similar postoperative mortality and incidence of complications. Simultaneous operation, i.e., hepatectomy combined with splenectomy and pericardial devascularization, was safe and feasible, as demonstrated by previous clinical studies conducted in China and abroad (15,16), and it was the most effective method among all the treatments for HCC with PHT and hypersplenism (17,18). However, its operation indication should be held strictly, particularly in laparoscopy, due the great difficulties of surgery (19,20). Furthermore, this is a technical challenge and is now demonstrated that in specialized centers there is no more difference in morbidity and mortality for HCC location (21). However, studies on whole laparoscopy were limited in the field of hepatectomy combined with splenectomy and pericardial devascularization.
Eligible patients were selected based on liver function and the difficulty of hepatectomy. Child class was the most commonly applied index of liver function assessment, which was the basis for any HCC treatment. Generally, patients with Child class A-B could tolerate surgical treatment, while patients with Child class C should only receive non-surgical treatment. In hepatectomy, only small-sized tumors (tumor size <5 cm) that are located on the edge of liver, or confined to certain liver lobe or adjacent segment, can be removed via lobectomy, segmentectomy or partial hepatectomy, due to the limitations of laparoscopy. In patients with preoperative hypersplenism, and moderate and severe varices in the esophagus and fundus of the stomach, a number of factors should be considered during the operation, particularly in resection of megalosplenia. Splenic artery ligation should be conducted first. The surgeon cannot free and detach the spleen until its volume has become smaller and the tension has decreased. Upon dissociation of perisplenic ligaments, the splenic pedicle should be removed with an Endo-Linear stapler. The excised spleen should be detached and placed in in specimen bag, and then removed in order not to lengthen the operative incision. In addition, removing the spleen allows more space for conducting pericardial devascularization in the abdominal cavity. Ultrasound knife and biological clip should be used for cutting the pericardial blood vessel under laparoscopy, which benefits the exposure of varices in the upper esophagus. Lastly, hepatectomy should be finished under laparoscopy. Since advanced cirrhosis always appears in patients with HCC accompanied by PHT, hepatic portal interdiction should be avoided in hepatectomy. It is important to control bleeding when detaching the liver parenchyma. In the present study, ultrasound knife was applied for accurate operation; biological clip and Hem-O-Lok were used for occlusion of wide vessels; and bipolar coagulation was employed for bleeding control. Although the operation shows great superiority for the treatment of PHT with HCC, splenectomy and porta-azygous disconnection are palliative therapies, which could only prevent gastrointestinal bleeding and improve liver function, while having no effect on liver cirrhosis. The recurrence rate of HCC remains high among patients with liver cirrhosis and HCC. Consequently, liver transplant should be advocated among patients for better therapeutic effects, provided the patients can afford it and there are suitable donors.
In this study, combined laparoscopic surgery were used in the treatment of HCC with PHT. The operation time and volume of blood loss were 336±18 min and 337±351 mL, respectively. The time for diet intake, drainage time and duration of hospital stay were 3.5±0.5, 7.3±1.0 and 13.6±3.6 days, respectively. The postoperative complications included 1 patient with anastomosis-site bleeding, 2 patients with abdominal effusion and 3 patients with portal vein thrombosis, which were treated conservatively. The overall survival rates at 1 and 3 years were 100% (16/16) and 87.5% (14/16), respectively. The recurrence-free survival rates at 1 and 3 years were 87.5% (14/16) and 62.5% (10/16), respectively. Compared with present studies, Harada et al. (13) reported that five-year recurrence-free survival in patients with HCC and PHT was significantly better in the laparoscopic liver resection than in the RFA group and there was no significant difference in the postoperative complication rate between laparoscopic liver resection and RFA group. The results also showed that laparoscopic liver resection may be a feasible treatment for patients with HCC and PHT. Meanwhile, Bai et al. (22) reported that Synchronous laparoscopic hepatectomy combined with splenectomy is safe and feasible for the treatment of HCC associated with PHT with an exact curative effect.
In summary, with the development of laparoscopic surgery, laparoscopy has been widely accepted for the treatment of primary HCC accompanied by PHT and hypersplenism. Reasonable evaluation, appropriate treatment and strict operation indication prior to operation are indispensable notwithstanding skillful manipulation is possessed. In addition, it is necessary to manipulate and cooperate closely, and to reduce surgical bleeding and postoperative complications as much as possible. Therefore, simultaneous hepatectomy, splenectomy and pericardial devascularization appears to be an effective treatment for primary HCC accompanied by PHT and hypersplenism. Laparoscopy may be gradually recognized and accepted by patients and healthcare workers.
Acknowledgments
Funding: The work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.01.23). The authors have no conflicts of interest to declare.
Ethics Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All experiments were ethically approved by the Research Ethics Committee of Southwest Hospital Affiliated to Third Military Medical University (Chongqing, China). Written informed consent was obtained from the patients regarding the publication of the case details.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Han H, Deng H, Han T, et al. Association Between Hepatocellular Carcinoma and Type 2 Diabetes Mellitus in Chinese Hepatitis B Virus Cirrhosis Patients: A Case-Control Study. Med Sci Monit 2017;23:3324-34. [Crossref] [PubMed]
- Lu Y, Sui J, Liu Y, et al. Association between hypoxia-inducible factor-1alpha gene polymorphisms and risk of chronic hepatitis B and hepatitis B virus-related liver cirrhosis in a Chinese population: a retrospective case-control study. Gene 2015;564:96-100. [Crossref] [PubMed]
- Yang F, Ma LT, Cao GW. Hepatocellular carcinoma: co - evolution of hepatocytes and hepatitis B virus. Zhonghua Gan Zang Bing Za Zhi 2017;25:321-4. [PubMed]
- Xu JM. Current treatment in advanced hepatocellular carcinoma and prospects for immuno-oncology therapy. Zhonghua Zhong Liu Za Zhi 2017;39:561-5. [PubMed]
- Kulik L, Heimbach JK, Zaiem F, et al. Therapies for patients with hepatocellular carcinoma awaiting liver transplantation: A systematic review and meta-analysis. Hepatology 2018;67:381-400. [Crossref] [PubMed]
- Kawano Y, Murata S, Taniai N, et al. Interventional Treatment of Severe Portal Vein Thrombosis after Living-Donor Liver Transplantation. J Nippon Med Sch 2016;83:206-10. [Crossref] [PubMed]
- Qiu B, Li K, Dong X, et al. Transjugular Intrahepatic Portosystemic Shunt for Portal Hypertension in Hepatocellular Carcinoma with Portal Vein Tumor Thrombus. Cardiovasc Intervent Radiol 2017;40:1372-82. [Crossref] [PubMed]
- Rich N, Tanriover B, Singal AG, et al. Outcomes of Simultaneous Liver Kidney Transplantation in Patients With Hepatocellular Carcinoma. Transplantation 2017;101:e12-9. [Crossref] [PubMed]
- Zhong JH, Li LQ. Portal hypertension should not be a contraindication of hepatic resection to treat hepatocellular carcinoma with compensated cirrhosis. Hepatology 2015;62:977-8. [Crossref] [PubMed]
- Zhao JB, Feng C, Zhu QH, et al. Transjugular intrahepatic portosystemic shunt with covered stents for hepatocellular carcinoma with portal vein tumor thrombosis. World J Gastroenterol 2014;20:1602-7. [Crossref] [PubMed]
- Hanazaki K, Kajikawa S, Adachi W, et al. Portal vein thrombosis may be a fatal complication after synchronous splenectomy in patients with hepatocellular carcinoma and hypersplenism. J Am Coll Surg 2000;191:341-2. [Crossref] [PubMed]
- Goumard C, Komatsu S, Brustia R, et al. Technical feasibility and safety of laparoscopic right hepatectomy for hepatocellular carcinoma following sequential TACE-PVE: a comparative study. Surg Endosc 2017;31:2340-9. [Crossref] [PubMed]
- Harada N, Maeda T, Yoshizumi T, et al. Laparoscopic Liver Resection Is a Feasible Treatment for Patients with Hepatocellular Carcinoma and Portal Hypertension. Anticancer Res 2016;36:3489-97. [PubMed]
- Sugawara Y, Yamamoto J, Shimada K, et al. Splenectomy in patients with hepatocellular carcinoma and hypersplenism. J Am Coll Surg 2000;190:446-50. [Crossref] [PubMed]
- Duan YF, Li XD, Sun DL, et al. A preliminary study on surgery for hepatocellular carcinoma patients with portal hypertension. Am J Surg 2015;210:129-33. [Crossref] [PubMed]
- Sun L, Zhou H, Gu L, et al. Effects of surgical procedures on the occurrence and development of postoperative portal vein thrombosis in patients with cirrhosis complicated by portal hypertension. Int J Surg 2015;16:31-5. [Crossref] [PubMed]
- Hu Q, Takeishi K, Yamashita Y, et al. Splenectomy Followed by Hepatectomy for Hepatocellular Carcinoma with Hypersplenism and Portal Hypertension Caused by Macroglobulinemia. Anticancer Res 2015;35:4077-81. [PubMed]
- Choi SB, Kim HJ, Song TJ, et al. Influence of clinically significant portal hypertension on surgical outcomes and survival following hepatectomy for hepatocellular carcinoma: a systematic review and meta-analysis. J Hepatobiliary Pancreat Sci 2014;21:639-47. [Crossref] [PubMed]
- Hu K, Lei P, Yao Z, et al. Laparoscopic RFA with splenectomy for hepatocellular carcinoma. World J Surg Oncol 2016;14:196. [Crossref] [PubMed]
- Ohno T, Furui J, Hashimoto T, et al. Simultaneous laparoscopic hand-assisted hepatectomy and splenectomy for liver cancer with hypersplenism: report of a case. Surgery Today 2011;41:444-7. [Crossref] [PubMed]
- Levi SG, Ettorre GM, Aldrighetti L, et al. Laparoscopic liver resection of hepatocellular carcinoma located in unfavorable segments: a propensity score-matched analysis from the I Go MILS (Italian Group of Minimally Invasive Liver Surgery) Registry. Surg Endosc 2018. [Epub ahead of print].
- Bai DS, Zhao W, Jiang G, et al. Synchronous laparoscopic hepatectomy combined with splenectomy for the treatment of hepatocellular carcinoma associated with cirrhotic portal hypertensive hypersplenism. Chin J Dig Surg 2013;14:750-54.


