
Serum biomarkers of inflammation for diagnosis of prostate cancer in patients with nonspecific elevations of serum prostate specific antigen levels
Introduction
Prostate cancer (PC) is a major health concern worldwide, being the second most common neoplasm and sixth cause of cancer-related death in the entire world (1). Some studies have found an association between inflammatory biomarkers and PC (2-6).
Serum lactate dehydrogenase (LDH) and C-reactive protein (CRP) are biomarkers of inflammation commonly used in medicine. CRP is an acute phase protein and a very sensitive marker of inflammation and tissue damage. It is synthesized mainly in the liver in response to interleukins. Levels of serum CRP can remain elevated in chronic inflammatory processes and cancer (6,7). LDH interconverts pyruvate and lactate at the end of the glycolytic pathway using NAD+ as a cofactor and are present in all tissues. LDH exists in five major isoenzymes, numbered LDH-1 through LDH-5. Serum total LDH is the sum of all the isoenzymes. Concentration of serum LDH can increase in many inflammatory processes and cancer (8).
The gold standard tools currently applied for the diagnosis of PC include the serum total prostate specific antigen (PSA), the digital rectal examination, and the ultrasound-guided systematic prostate biopsy sampling. Serum PSA has become the most clinically useful tumour marker for the diagnosis and subsequent monitoring of PC. The patients are selected for prostate biopsy on the basis of serum PSA levels. Patients with nonspecific elevations of serum PSA levels, values in the intermediate range of 4 to 10 ng/mL, provide less diagnostic certainty, resulting in high false-positive rates and a large number of unnecessary biopsies. The free-to-total serum prostate specific antigen ratio (%fPSA) has been proposed to differentiate benign from malignant prostate disease, improving specificity while maintaining sensitivity, in these patients (9).
The aim of this study was to evaluate the utility of serum LDH and CRP levels for diagnosis of PC in men with serum PSA levels in the intermediate range of 4 to 10 ng/mL.
Methods
This is a prospective and descriptive study whose methodology has been authorized by the Cadiz Ethics of Research Committee and all the participants have signed the informed consent.
Patients
We studied asymptomatic men from Puerto Real University Hospital with no known history of PC and serum PSA levels in the intermediate range of 4 to 10 ng/mL, who underwent 12-core transrectal ultrasound guided prostate biopsy for the first time from 2014 to 2016. Patients with other inflammatory pathologies (autoimmune diseases, hepatitis, pancreatitis, cirrhosis, infectious mononucleosis, sepsis, tumours) or patients with haemolysed samples that could elevate serum biomarkers of inflammation levels were excluded. Patients were classified into two groups according to the diagnosis of prostate biopsy: PC and NOT PC patients.
Biomarkers
Prior to biopsy and after obtaining an informed consent, blood specimens were drawn by venipuncture in gel separator serum tubes and centrifuged at 4,000 rpm for 5 minutes. The measurement of the haemolytic index (HI) were determined by colorimetric method on Hitachi Modular cobas c 702 (Roche Diagnostics, Basel, Switzerland) and just the non-haemolysed samples, those with a HI below 50 units, were included in the study.
The following serum biomarkers were measured: PSA and free-PSA by electrochemiluminescence immunoassay on Hitachi Modular E-170 analyzer (Roche Diagnostics, Basel, Switzerland); LDH by enzymatic photometric method according to the International Federation of Clinical Chemistry and CRP by immunoturbidimetric test with monoclonal anti-CRP antibodies on Hitachi Modular cobas c 702 analyzer (Roche Diagnostics, Basel, Switzerland). The reference range in serum for LDH and CRP is 135–225 U/L and <5.0 mg/L respectively. The %fPSA was calculated using the following formula: (free-PSA/PSA) ×100 (%).
The prostate volume was determined by transrectal ultrasound using the longitudinal and transverse diameters (10): Prostate volume = [(longitudinal diameter)2 × transverse diameter]/2.
Statistical analysis
The data obtained was processed by the statistical program Medcalc®, where P<0.05 was considered as statistically significant. D’Agostino-Pearson test was used to determine the type variable distribution. Descriptive statistics of the variables with normal distribution were expressed with the range, mean and standard deviation, and variables with non-Gaussian distribution with the range, median and interquartile range. The correlation between variables with normal distribution were analyzed using the Pearson correlation coefficient, and between variables with non-Gaussian distribution using the Spearman rho. The comparison between groups was performed using analysis of variance test for normally distributed variables and Mann-Whitney test for variables with non-Gaussian distribution. Logistic regression was used for develop a probabilistic model to predict patients with PC and determine the importance of each biomarker by calculating the odds ratio. The diagnostic accuracy was determined using receiver operating characteristic curves (ROC), calculating the area under the ROC curve (AUC) and the optimal cut-off point with its corresponding sensitivity and specificity. The optimal cut-off point was that which had the highest sensitivity and specificity, which correctly classified the largest number of patients.
Results
We studied 232 patients with ages between 43 and 98 years old (median =72), 200 NOT PC patients (86.2%) and 32 PC patients (13.8%). All PC patients had no metastasis, 30 of PC patients showed Gleason score ≤7, and just two with Gleason score =8. All the variables studied followed a non-Gaussian distribution. No statistically significant differences were found between PC and NOT PC patients according to the age, nor was there a significant correlation between the age of the patients and the variables analyzed (P>0.05).
Descriptive statistics of prostate volume and serum PSA, %fPSA, LDH and CRP levels in PC and NOT PC patients are showed in Table 1. No statistical correlation was found between prostate volume or %fPSA and LDH or CRP (P>0.05). A low intensity correlation was obtained between LDH and CRP, Spearman rank correlation coefficient (rho) =0.178 (P=0.0068).
Table 1
| Biomarker | PC | n | Range | Median (95% CI) | IR | P value |
|---|---|---|---|---|---|---|
| Prostate volume (cm3) | 0 | 200 | 16.0–96.9 | 59.0 (33.6–81.0) | 44.0 | >0.05* |
| 1 | 32 | 14.0–78.5 | 52.5 (34.7–68.0) | 26.5 | ||
| PSA (ng/mL) | 0 | 200 | 4.01–9.95 | 5.58 (5.19–5.70) | 2.89 | >0.05* |
| 1 | 32 | 4.05–9.99 | 6.62 (5.53–7.01) | 1.80 | ||
| %fPSA (%) | 0 | 200 | 4.77–58.81 | 20.46 (16.96–22.56) | 14.90 | <0.0001* |
| 1 | 32 | 3.80–21.31 | 8.87 (6.97–13.29) | 7.50 | ||
| LDH (U/L) | 0 | 200 | 121–543 | 206 (195–240) | 122 | 0.0048* |
| 1 | 32 | 138–844 | 298 (194–449) | 272 | ||
| CRP (mg/L) | 0 | 200 | 0.2–363.3 | 8.2 (6.5–12.5) | 35.1 | >0.05* |
| 1 | 32 | 0.7–247.2 | 7.6 (2.9–47.5) | 75.0 |
*, U Mann-Whitney test. PC, prostate cancer; CI, confidence interval; IR, interquartile range; PSA, serum total prostate specific antigen; %fPSA, free-to-total serum prostate specific antigen ratio; LDH, serum lactate dehydrogenase; CRP, serum C-reactive protein; 0, NOT PC patients; 1, PC patients.
In this study, prostate volume, serum PSA and CRP levels were not statistically significantly to differentiate between PC and NOT PC patients (P>0.05). Serum LDH levels and %fPSA values were included in the probabilistic model to predict patients with PC by logistic regression. The odds ratios were 0.8530 [95% confidences interval (CI): 0.7933–0.9173] and 1.0071 (95% CI: 1.0033–1.0108); and coefficients were −0.1589 (P<0.0001) and 0.0070 (P=0.0002) for %fPSA and serum LDH, respectively. The probabilistic model to predict patients with PC was: LDH + %fPSA (probability %) = 100 × (1+ e−Z)−1; (Z =0.0070 × LDH –0.1589× %fPSA –1.4898).
The ROC curves of probabilistic model, serum LDH levels and %fPSA values to differentiate between PC and NOT PC patients are compared in Figure 1. AUC, optimal cut-off value, sensitivity and specificity for the diagnosis of PC using probabilistic model, serum LDH levels and %fPSA values are shown in Table 2.
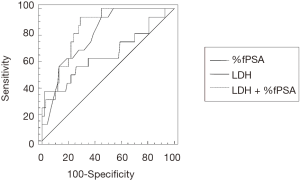
Table 2
| Biomarker | AUC (95% CI) | Cut-off | Sensitivity (95% CI) (%) | Specificity (95% CI) (%) |
|---|---|---|---|---|
| %fPSA | 0.802 (0.745–0.851) (P<0.0001) | 17.42% | 93.7 (79.2–99.1) | 55.0 (47.8–62.0) |
| LDH | 0.657 (0.592–0.718) (P=0.0048) | 436 U/L | 37.5 (21.1–56.3) | 98.0 (95.0–99.4) |
| LDH + %fPSA | 0.844 (0.797–0.893) (P<0.0001) | 13.62% | 93.7 (79.2–99.1) | 71.0 (64.2–77.2) |
ROC, receiver operating characteristic; AUC, area under the receiver operating characteristic curves; CI, confidence interval; %fPSA, free-to-total serum prostate specific antigen ratio; LDH, serum lactate dehydrogenase; LDH +% fPSA: probabilistic model [probability (%) =100× (1+ e−Z)−1; Z =0.0070× LDH –0.1589× %fPSA –1.4898].
Discussion
In this study, the prostate volume and CRP were slightly higher in NOT PC patients, although they were not useful to differentiate benign from malignant prostate disease in these patients. In contrast, serum LDH levels were significantly higher in the PC patients and %fPSA values were very higher in the NOT PC patients (Table 1).
No correlation was found between prostate volume and serum biomarkers of inflammation. There was a very low correlation between serum levels of LDH and CRP (rho =0.178), so they can be considered independent biomarkers.
Serum CRP level appears to be an independent prognostic factor of PC (11,12). High concentrations of serum CRP have been associated with shorter overall survival in patients with castration-refractory PCs (13) and with a poor prognosis in PC patients undergoing radiotherapy (14-16), however high serum CRP levels have not been associated with an increased risk of PC (3,6,7,17-22). In this study, serum CRP levels were not useful to differentiate benign from malignant prostate disease in patients with serum PSA levels in the intermediate range of 4 to 10 ng/mL. This result may be due to the fact that serum CRP is a biomarker of inflammation with high sensitivity but low specificity, so NOT PC patients can have elevated serum CRP due to other diseases (including benign prostatic hypertrophy) resulting in a large number of false positives.
Some studies have found an association between LDH and PC: the activity and protein level of mitochondrial LDH isomers (D and L) are higher in tumours cells than in normal cells (23,24); LDH 5 isoenzyme overexpression is significantly linked to highly proliferating prostate carcinomas and with biochemical failure and local relapse following radiotherapy (25); serum LDH levels was suggested to be prognostic indicator in PC patients with bone metastasis (26); and recently we have proposed the combination of serum LDH levels and %fPSA values for the diagnosis of PC using a multivariable score, but a logistic regression analysis was not performed to develop a probabilistic model (27). In this study, serum LDH levels and %fPSA values were independent predictors for diagnosis of PC. Serum LDH levels showed high specificity with low sensitivity and %fPSA values had high sensitivity with low specificity for the diagnosis of PC in men with intermediate serum PSA levels. Probabilistic model to predict patients with PC using serum LDH levels and %fPSA values improved accuracy, exhibiting 93.7% sensitivity and 71.0% specificity. Probabilistic model increased the specificity by 16% compared to using %fPSA alone (Table 2). High serum LDH levels in PC patients may be because the tumour cells have high activity of glycolysis, increase glucose consumption and lactate release, requiring higher enzymatic activity of LDH independently from the presence of oxygen (Warburg effect). LDH could be a possible pharmacological target in cancer therapy.
In other studies, chronic inflammation of multiple etiologies was a risk factor for PC (6), and serum CRP levels were well-correlated with serum PSA levels in PC patients, suggesting a potential correlation between prostate inflammation and PC (21). In this study, no statistical correlation was found between %fPSA values and serum LDH or CRP levels (P>0.05).
The main limitation of this study is the low number of PC patients (n=32), further studies with larger number of patients are needed to confirm the utility of serum LDH for diagnosis of PC.
In conclusion, serum CRP levels were not useful to differentiate benign from malignant prostate disease, in contrast serum LDH levels could be used for diagnosis of PC in patients with serum PSA levels in the intermediate range of 4 to 10 ng/mL. A probabilistic model to predict patients with PC using serum LDH levels and %fPSA values can improve the diagnostic accuracy and reduce the false positive rate, avoiding unnecessary biopsies in patients with nonspecific elevations of serum PSA levels.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.01.31). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This is a prospective and descriptive study whose methodology has been authorized by the Cadiz Ethics of Research Committee and all the participants have signed the informed consent (Prostate cancer 20.10.2016).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Center MM, Jemal A, Lortet-Tieulent J, et al. International variation in prostate cancer incidence and mortality rates. Eur Urol 2012;61:1079-92. [Crossref] [PubMed]
- Sun Z, Ju Y, Han F, et al. Clinical implications of pretreatment inflammatory biomarkers as independent prognostic indicators in prostate cancer. J Clin Lab Anal 2018;32: [Crossref] [PubMed]
- De Marzo AM, Platz EA, Sutcliffe S, et al. Inflammation in prostate carcinogenesis. Nat Rev Cancer 2007;7:256-69. [Crossref] [PubMed]
- Sciarra A, Di Silverio F, Salciccia S, et al. Inflammation and chronic prostatic diseases: evidence for a link? Eur Urol 2007;52:964-72. [Crossref] [PubMed]
- Cheng I, Witte JS, Jacobsen SJ, et al. Prostatitis, sexually transmitted diseases, and prostate cancer: the California Men’s Health Study. PLoS One 2010;5:e8736. [Crossref] [PubMed]
- St Hill CA, Lutfiyya MN. An epidemiological analysis of potential associations between C-reactive protein, inflammation, and prostate cancer in the male US population using the 2009-2010 National Health and Nutrition Examination Survey (NHANES) data. Front Chem 2015;3:55. [Crossref] [PubMed]
- Allin KH, Bojesen SE, Nordestgaard BG. Baseline C-reactive protein is associated with incident cancer and survival in patients with cancer. J Clin Oncol 2009;27:2217-24. [Crossref] [PubMed]
- Gallo M, Sapio L, Spina A, et al. Lactic dehydrogenase and cancer: an overview. Front Biosci (Landmark Ed) 2015;20:1234-49. [Crossref] [PubMed]
- Aus G, Abbou CC, Bolla M, et al. European Association of Urology. EAU guidelines on prostate cancer. Eur Urol 2005;48:546-51. [Crossref] [PubMed]
- Rodríguez-Patrón Rodríguez R, Mayayo Dehesa T, Burgos Revilla FJ, et al. The role of prostate volume in ultrasound guided transrectal prostate biopsy: is it as important as a marker as PSA? Arch Esp Urol 2005;58:903-13. [PubMed]
- Pond GR, Armstrong AJ, Wood BA, et al. Ability of C-reactive protein to complement multiple prognostic classifiers in men with metastatic castration resistant prostate cancer receiving docetaxel-based chemotherapy. BJU Int 2012;110:E461-8. [Crossref] [PubMed]
- Liu ZQ, Chu L, Fang JM, et al. Prognostic role of C-reactive protein in prostate cancer: a systematic review and meta-analysis. Asian J Androl 2014;16:467-71. [Crossref] [PubMed]
- Beer TM, Lalani AS, Lee S, et al. C-reactive protein as a prognostic marker for men with androgen-independent prostate cancer: results from the ASCENT trial. Cancer 2008;112:2377-83. [Crossref] [PubMed]
- Graff JN, Beer TM. The role of C-reactive protein in prostate cancer. Cancer 2013;119:3262-4. [Crossref] [PubMed]
- Graff JN, Beer TM, Liu B, et al. Pooled Analysis of C-Reactive Protein Levels and Mortality in Prostate Cancer Patients. Clin Genitourin Cancer 2015;13:e217-21. [Crossref] [PubMed]
- Thurner EM, Krenn-Pilko S, Langsenlehner U, et al. The elevated C-reactive protein level is associated with poor prognosis in prostate cancer patients treated with radiotherapy. Eur J Cancer 2015;51:610-9. [Crossref] [PubMed]
- Platz EA, De Marzo AM, Erlinger TP, et al. No association between pre-diagnostic plasma C-reactive protein concentration and subsequent prostate cancer. Prostate 2004;59:393-400. [Crossref] [PubMed]
- Siemes C, Visser LE, Coebergh JW, et al. C-reactive protein levels, variation in the C-reactive protein gene, and cancer risk: the Rotterdam Study. J Clin Oncol 2006;24:5216-22. [Crossref] [PubMed]
- Heikkilä K, Silander K, Salomaa V, et al. C-reactive protein-associated genetic variants and cancer risk: findings from FINRISK 1992, FINRISK 1997 and Health 2000 studies. Eur J Cancer 2011;47:404-12. [Crossref] [PubMed]
- Pierce BL, Biggs ML, DeCambre M, et al. C-reactive protein, interleukin-6, and prostate cancer risk in men aged 65 years and older. Cancer Causes Control 2009;20:1193-203. [Crossref] [PubMed]
- Chang CC, Lin AT, Chen KK, et al. The Significance of Plasma C-reactive Protein in Patients With Elevated Serum Prostate-specific Antigen Levels. Urol Sci 2010;21:88-92. [Crossref]
- Schnoeller TJ, Steinestel J, Steinestel K, et al. Do preoperative serum C-reactive protein levels predict the definitive pathological stage in patients with clinically localized prostate cancer? Int Urol Nephrol 2015;47:765-70. [Crossref] [PubMed]
- De Bari L, Chieppa G, Marra E, et al. L-lactate metabolism can occur in normal and cancer prostate cells via the novel mitochondrial L-lactate dehydrogenase. Int J Oncol 2010;37:1607-20. [PubMed]
- de Bari L, Moro L, Passarella S. Prostate cancer cells metabolize d-lactate inside mitochondria via a D-lactate dehydrogenase which is more active and highly expressed than in normal cells. FEBS Lett 2013;587:467-73. [Crossref] [PubMed]
- Koukourakis MI, Giatromanolaki A, Panteliadou M, et al. Lactate dehydrogenase 5 isoenzyme overexpression defines resistance of prostate cancer to radiotherapy. Br J Cancer 2014;110:2217-23. [Crossref] [PubMed]
- Naruse K, Yamada Y, Aoki S, et al. Lactate dehydrogenase is a prognostic indicator for prostate cancer patients with bone metastasis. Hinyokika Kiyo 2007;53:287-92. [PubMed]
- Santotoribio JD, Cañavate-Solano C, Garcia-de la Torre A, et al. Serum lactate dehydrogenase in combination with free-to-total serum prostate specific antigen ratio for diagnosis of prostate cancer. Clin Lab 2014;60:1055-8. [Crossref] [PubMed]


