
STX4 expression is associated with classification, clinical stage and lymphatic metastasis in ovarian cancer
Introduction
Ovarian cancer (OC) is one of the most common malignant tumors in female reproductive system. In China (1), OC is the most frequent cause of cancer-related death from gynecological malignancies, and the majority of patients are diagnosed at an advanced stage (2). Furthermore, 55–75% of the patients relapsed after surgery and standard postoperative chemotherapy and radiotherapy, and are rarely cured after recurrence. The 5-year survival rate for OC patients at stage IIIC and IV is 29% and 13%, respectively (3,4). Therefore, it is urgently necessary to understand the molecular mechanism underlying recurrence and progression of OC and discover specific molecular markers for the diagnosis and treatment of OC. New treatments are needed for advanced and recurrent OC. More and more attention has been paid to immunotherapy, a new adjuvant therapy, to break the body’s immune tolerance to tumor and improve the immune response of the body’s immune system to cancer.
Metastasis, including local tumor growth, migration and invasion of cancer cells into lymphatic and blood vessels, survive and spread in the circulation, and extravasation and establishment of secondary colonies at distant sites (5), is responsible for more than 90% of cancer deaths. As a family of heterodimeric receptors for cell adhesion to the extracellular matrix (ECM), integrins, such as fibronectin, laminin, collagen and vitronectin (6), play critical roles in cell migration, cancer progression and metastasis. As transmembrane proteins, integrins are transported in vesicles and delivered to the cell surface by vesicular trafficking. Fusion of integrin-containing vesicles with the plasma membrane is the final step of integrin delivery. The SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) proteins, syntaxin (STX) 1, 2, 3 and 4, are localized in the plasma membrane to drive vesicle fusion. Studies have shown that the SNARE proteins play a fundamental role in the occurrence, development, invasion and metastasis of cancer cells, and more and more attention has been paid to them as therapeutic targets for various tumors (7,8). STX is one of the components of SNARE family, which consists of three groups of proteins, including synaptosomal-associated protein (SNAP), STX and vesicle-associated membrane protein (VAMP) (9-11). STX family proteins are composed of 16 members, including STX1, STX2, STX3 and STX4, which are localized in the plasma membrane. STXs have been linked to a variety of malignancies. For example, STX8 can affect epidermal growth factor receptor (EGFR) in glioblastoma. The signal transduction of growth factor is involved in tumor development process. Previous data have suggested that STX3- or STX4-dependent integrin trafficking is important in migration and survival of cancer cells, which may be valuable targets for cancer therapy (12).
However, only very few studies have established a correlation between STX4 expression and OC progression and differentiation. In the present study, we aimed to investigate the correlation between STX4 expression and clinicopathological features of epithelial OC patients. Our findings suggested that STX4-targeted treatment could be used as a potential therapeutic strategy for OC.
Methods
Clinical samples
All clinical tissue samples were obtained from the Third Affiliated Hospital of Soochow University (Changzhou, China) between January 2008 and January 2017. These specimens, including 80 cases of primary epithelial OC (five well-differentiated cases, eight moderately differentiated cases, 52 poorly differentiated cases and 15 undifferentiated cases in reports), one case of borderline epithelial OC and 12 cases of benign epithelial OC, were collected from patients who underwent surgery. Detailed clinicopathologic variables of the patients are summarized in Table 1. The study design was approved by the ethics committee of Soochow University (Changzhou, China; No. 2018046). Written informed consent was obtained from every patient.
Table 1
| Variables | Patients (n) | STX4 immunostaining score | Z/χ2 | P |
|---|---|---|---|---|
| T character | 4.315 | <0.001 | ||
| Benign tumors | 12 | 70.00±60.49 | ||
| Malignancy tumors | 80 | 185.18±69.20 | ||
| T classification | 2.418 | 0.016 | ||
| Serous adenocarcinoma | 69 | 169.41±79.90 | ||
| Mucinous adenocarcinoma | 7 | 90.00±70.30 | ||
| N metastasis | 2.069 | 0.039 | ||
| Y | 8 | 223.75±25.60 | ||
| N | 29 | 177.41±63.65 | ||
| Clinical stage | 2.325 | 0.020 | ||
| I | 19 | 157.11±65.92 | ||
| II/III/IV | 61 | 193.92±68.37 | ||
| Differentiation | 3.718 | 0.156 | ||
| Poorly differentiated | 52 | 208.73±48.66 | ||
| Moderately differentiated | 8 | 155.63±74.04 | ||
| Well-differentiated | 5 | 175.00±95.26 |
Due to statistical reasons, one borderline OC patient and two endometrioid adenocarcinoma patients were not included. STX4, syntaxin 4; SD, standard deviation; T, tumor; N, lymph node.
Immunohistochemistry
The primary antibodies used for immunohistochemistry were commercially available, including STX-4 antibody (ab77037, Abcams, CO, USA). Formalin-fixed, paraffin-embedded consecutive sections (4-µm thick) were heated at 85 °C for 2 h and then cooled at room temperature for 20 min. The slides were immersed in dimethylbenzene three times for deparaffinage 15 min each time and then consecutively hydrated in 100%, 95% and 75% ethanol for 5 min. For antigen retrieval, slides were heated at 125 °C for 5 min in 2% EDTA-citrate antigen retrieval solution (MVS-0099, Fuzhou Maixin Biotech. Co., Ltd., Fuzhou, China) in a pressure cooker. Subsequently, slides were rinsed with PBS (PBS-0061, Fuzhou Maixin Biotech. Co., Ltd., Fuzhou, China) for three times and then immersed in hydrogen peroxide at room temperature for 30 min to block endogenous peroxidase, followed by incubation with 3% BSA at 37 °C for 30 min to block nonspecific binding. Next, the slides were incubated with primary antibody against STX4 (1:200 diluted using antibody diluent) at 4 °C for 14 h. A MaxVisionTM rapid immunohistochemistry kit (KIT-5020, Fuzhou Maixin Biotech. Co., Ltd., Fuzhou, China) was used in the present study, and the binding process of secondary antibody was carried out according to the manufacturer’s instructions. A DAB substrate kit (DAB-0031, Fuzhou Maixin Biotech. Co., Ltd., Fuzhou, China) was employed and its staining process was conducted according to the manufacturer’s instructions. After staining, the sections were counterstained using hematoxylin, followed by dehydration through ethanol and xylene.
Evaluation of immunohistochemical staining and statistical analysis
All slides were independently examined by two senior pathologists who were blinded to the clinical parameters of patients. The immunostaining density of STX4 was assessed according to the H-score method: H-score = (% unstained tumor cells ×0) + (% weakly stained tumor cells ×1) + (% moderately stained tumor cells ×2) + (% strongly stained tumor cells ×3). The H-scores ranged from 0 (100% negative tumor cells) to 300 (100% strong staining tumor cells). Results from the two pathologists were averaged and used in the statistical analysis. All data were expressed as the mean ± standard deviation (M ± SD). Statistical analysis was performed using SPSS version 22.0 (SPSS Inc., St. Chicago, IL, USA). The correlation among all the groups was compared by Kruskal-Wallis test and Mann-Whitney U test. P<0.05 was considered statistically significant.
Results
Immunohistochemistry
STX4 staining was observed in various proportions of tumor cells and localized in cell membrane (brown granular). In all 93 cases, the average H-score of STX4 expression in benign patients and OC patients was 70.00±60.49 and 185.18±69.20, respectively (u=4.315, P<0.05; Table 1 and Figure 1). The H-score of STX4 for one patient with borderline epithelial tumor, was 265. In all the patients with epithelial OC, 69 cases (86.25%) were serous cystadenocarcinoma, and seven cases were mucinous cystadenocarcinoma (8.75%), with a staining score of 169.41±79.90 and 90.00±70.30, respectively (u=2.418, P<0.05; Table 1 and Figure 2). The staining H-score of STX4 in patients with endometrioid carcinoma (two cases, 2.5%) was 167.50±3.54, and there was no statistical significance between endometrioid carcinoma and serous cystadenocarcinoma (P>0.05; Figure 2).
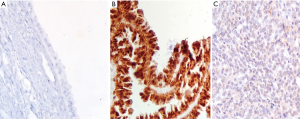
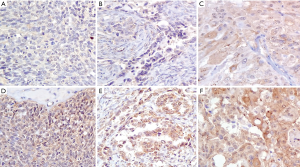
Relationship with clinicopathological variables
In the 80 epithelial OC patients, 19 cases (23.75%) were in early stage (stage I), and 61 cases were in late stage (76.25%), including stage II, III and IV. The staining score of STX4 in patients of early and late stages was 157.11±65.92 and 193.92±68.37, respectively (u=2.325, P<0.05). Additionally, the staining score of STX4 in stage II was 208.57±64.61, which was significantly different from that of stage I (P<0.05).
Among 80 epithelial OC patients, lymphadenectomy was performed on 37 cases, in which 29 patients (78.38%) had no lymphatic metastasis and 8 patients (21.62%) exhibited lymphatic metastasis, with a staining score of 177.41±63.65 and 223.75±25.60, respectively (u=2.069, P<0.05; Table 1).
In the 80 epithelial OC patients, 52 cases (65.00%) were poorly differentiated, eight cases (10.00%) were moderately differentiated, and five patients (6.25%) were well differentiated, with a staining score of 208.73±48.66, 155.63±74.04 and 175.00±95.26, respectively (Z=3.718, P>0.05; Table 1). However, there was no statistical significance among these subgroups.
Discussion
Study has shown that depletion of STX4 reduces the proportion of cells forming active invadopodia by >50%. STX4 and synaptosomal-associated protein 23 (SNAP23) are associated with vesicle-associated membrane protein 7 (VAMP7) possibly in a complex, and function to deliver membrane type-1 matrix metalloproteinase (MT1-MMP) to invadopodia during degradation of ECM by MDA-MB-231 breast tumor cells (13). Recent models suggest that tumor cell invasion can be mediated by subcellular structures called invadopodia, which can facilitate MMP-mediated degradation of ECM (14). Evidence from in vivo studies has supported that invadopodia plays a role in the dissemination of tumor cell populations (15,16). Membrane trafficking of proteins to invadopodia is required for their formation and function in support of tumor cell invasion (17).
In HeLa cervical adenocarcinoma cells and PANC-1 pancreatic adenocarcinoma cells, depletion of STX4 reduces the cell surface expressions of α5β1 and α3β1 integrins, indicating that STX4 is involved in α5β1 and α3β1 trafficking to the cell surface (12). In addition, depletion of STX4 inhibits cell adhesion to fibronectin and chemotactic cell migration, and triggers apoptosis, suggesting that STX4-mediated integrin trafficking is important for migration and survival of cancer cells. In migrating cells, STX4 has been shown to associate with lipid rafts, which are concentrated at the leading edge to establish front-rear polarity (18,19). In macrophages, STX4 forms a complex with VAMP3 and SNAP-23 to deliver α5β1 integrin to the cell surface (20). Study has indicated that cells lacking STX4 have multiple actin-rich spindly protrusions, and they are unable to exocytose VAMP3-positive recycling endosomes. The multiple sites of protrusion in cells that lack STX4 indicate a defect in cell polarity in these cells (21).
Immunocytochemistry reveals that transient transfection of AGS cells, a human gastric epithelial cancer cell line, with dominant-negative mutant STX 4 decreases the expression of plasma membrane MT1-MMP. MMPs also play promoting roles in cancer invasion and metastasis (2). For example, MMPs regulate the degradation of ECM surrounding the tumor surface (22) and enhance neovascularization during invasion and metastasis of cancer (23). In neuronal cells, STX4 has recently been shown to define a site of exocytosis for AMPA receptor-containing recycling compartments at the tips of dendritic spines that direct membrane fusion and regulate postsynaptic plasticity (24). The location of STX4 to the basolateral membrane in polarized epithelial cells is thought to regulate delivery of cargo specifically located on the basolateral membrane (25,26). In all above-mentioned cases, STX4 plays a fundamental role in the polarized delivery of vesicles to specific locations on the plasma membrane, suggesting that STX4 acts to define sites of focal exocytosis in the plasma membrane. Taken together, these results suggest that STX4/SNAP23 is a key regulator in focal exocytosis of recycling compartments in the plasma membrane both in macrophages and other cell types (21).
Our study demonstrated that STX4 was positively expressed in OC tissues. Meanwhile, the expression of STX4 was positively correlated with the clinical stage of human OC, classification and node metastasis, suggesting that STX4 was involved in the occurrence and development of OC. Based on our findings, we speculated that the activation of STX4 might promote the proliferation and invasion of cancer cells, leading the development of OC. Since the operation in 80 of the 90 cases we analyzed was performed during the last 3 years, the information on overall survival (OS) and disease-free survival (DFS) could not be determined. Therefore, the relationship between STX4 expression and OS or DFS was not assessed in the present study.
Collectively, we showed that STX4 was over-expressed in OC, and such abnormal expression of STX4 promoted the progression of OC, indicating that the invasiveness of tumor cells might be possibly inhibited by targeting STX4 in cancer cells. Taken together, our findings provided an experimental foundation for therapeutic strategy, and suggested that RNA interference technology or other relevant methods might be used to decrease the expression of STX4 and suppress the invasion of OC cells. However, the effects of STX4 on the clinical outcomes of OC needed to be further investigated, including both in vitro experiment and animal model.
Acknowledgments
Funding: Project supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.02.11). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study design was approved by the ethics committee of Soochow University (Changzhou, China; No. 2018046). Written informed consent was obtained from every patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Li J, Yang W, Wu X. Prognostic factors and role of salvage surgery in chemorefractory ovarian germ cell malignancies: a study in Chinese patients. Gynecol Oncol 2007;105:769-75. [Crossref] [PubMed]
- Kim PS, Djazayeri S, Zeineldin R. Novel nanotechnology approaches to diagnosis and therapy of ovarian cancer. Gynecol Oncol 2011;120:393-403. [Crossref] [PubMed]
- Chen SS, Michael A, Butler-Manuel SA. Advances in the treatment of ovarian cancer: a potential role of antiinflammatory phytochemicals. Discov Med 2012;13:7-17. [PubMed]
- Chi DS, Eisenhauer EL, Zivanovic O, et al. Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol Oncol 2009;114:26-31. [Crossref] [PubMed]
- Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol 2005;17:548-58. [Crossref] [PubMed]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell 2002;110:673-87. [Crossref] [PubMed]
- Meng J, Wang J. Role of SNARE proteins in tumourigenesis and their potential as targets for novel anti-cancer therapeutics. Biochim Biophys Acta 2015;1856:1-12. [PubMed]
- Collins LE, DeCourcey J, Soledad di Luca M, et al. An Emerging Role for SNARE Proteins in Dendritic Cell Function. Front Immunol 2015;6:133. [Crossref] [PubMed]
- Söllner T, Whiteheart SW, Brunner M, et al. SNAP receptors implicated in vesicle targeting and fusion. Nature 1993;362:318-24. [Crossref] [PubMed]
- Trimble WS, Cowan DM, Scheller RH. VAMP-1: a synaptic vesicle-associated integral membrane protein. Proc Natl Acad Sci U S A 1988;85:4538-42. [Crossref] [PubMed]
- Bennett MK, Calakos N, Scheller RH. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science 1992;257:255-9. [Crossref] [PubMed]
- Day P, Riggs KA, Hasan N, et al. Syntaxins 3 and 4 mediate vesicular trafficking of α5β1 and α3β1 integrins and cancer cell migration. Int J Oncol 2011;39:863-71. [PubMed]
- Williams KC, McNeilly RE, Coppolino MG. SNAP23, Syntaxin4, and vesicle-associated membrane protein 7 (VAMP7) mediate trafficking of membrane type 1-matrix metalloproteinase (MT1-MMP) during invadopodium formation and tumor cell invasion. Mol Biol Cell 2014;25:2061-70. [Crossref] [PubMed]
- Murphy DA, Courtneidge SA. The 'ins' and 'outs' of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Biol 2011;12:413-26. [Crossref] [PubMed]
- Clark ES, Brown B, Whigham AS, et al. Aggressiveness of HNSCC tumors depends on expression levels of cortactin, a gene in the 11q13 amplicon. Oncogene 2009;28:431-44. [Crossref] [PubMed]
- Lohmer LL, Kelley LC, Hagedorn EJ, et al. Invadopodia and basement membrane invasion in vivo. Cell Adh Migr 2014;8:246-55. [Crossref] [PubMed]
- Poincloux R, Lizárraga F, Chavrier P. Matrix invasion by tumour cells: a focus on MT1-MMP trafficking to invadopodia. J Cell Sci 2009;122:3015-24. [Crossref] [PubMed]
- Lafont F, Verkade P, Galli T, et al. Raft association of SNAP receptors acting in apical trafficking in Madin-Darby canine kidney cells. Proc Natl Acad Sci U S A 1999;96:3734-8. [Crossref] [PubMed]
- Chamberlain LH, Gould GW. The vesicle- and target-SNARE proteins that mediate Glut4 vesicle fusion are localized in detergent-insoluble lipid rafts present on distinct intracellular membranes. J Biol Chem 2002;277:49750-4. [Crossref] [PubMed]
- Veale KJ, Offenhäuser C, Whittaker SP, et al. Recycling endosome membrane incorporation into the leading edge regulates lamellipodia formation and macrophage migration. Traffic 2010;11:1370-9. [Crossref] [PubMed]
- Veale KJ, Offenhäuser C, Murray RZ. The role of the recycling endosome in regulating lamellipodia formation and macrophage migration. Commun Integr Biol 2011;4:44-7. [Crossref] [PubMed]
- Yana I, Seiki M. MT-MMPs play pivotal roles in cancer dissemination. Clin Exp Metastasis 2002;19:209-15. [Crossref] [PubMed]
- Hiraoka N, Allen E, Apel IJ, et al. Matrix metalloproteinases regulate neovascularization by acting as pericellular fibrinolysins. Cell 1998;95:365-77. [Crossref] [PubMed]
- Kennedy MJ, Davison IG, Robinson CG, et al. Syntaxin-4 defines a domain for activity-dependent exocytosis in dendritic spines. Cell 2010;141:524-35. [Crossref] [PubMed]
- Low SH, Chapin SJ, Wimmer C, et al. The SNARE machinery is involved in apical plasma membrane trafficking in MDCK cells. J Cell Biol 1998;141:1503-13. [Crossref] [PubMed]
- ter Beest MB, Chapin SJ, Avrahami D, et al. The role of syntaxins in the specificity of vesicle targeting in polarized epithelial cells. Mol Biol Cell 2005;16:5784-92. [Crossref] [PubMed]


