
Stereotactic body radiation therapy (SBRT) for non-small cell lung cancer (NSCLC): current concepts and future directions
Introduction
It is estimated that there will be over 226,000 new diagnoses and 159,000 deaths from lung cancer in the United States in 2014 (1). In fact, lung cancer deaths annually total more than deaths from breast, prostate, colon, brain and uterine cancer combined. Additionally, lung cancer is the leading cause of cancer mortality in both men and women worldwide, with estimated incidence rates of over 2 million cases annually, and death rates nearing 1.5 million annually (2,3). One major reason lung cancer continues to be such a major contributor to cancer deaths includes the combination of the late stage of diagnosis of the majority of cases, and the lack of an accepted, widely instituted screening program to detect early stage disease. At present, only about 15% of lung cancers are diagnoses at an early stage (stage I and II) and over half are diagnosed with metastatic disease (1).
Smoking has been well established as a leading risk factor for lung cancer. Despite the recent decreases in smoking rates in the US (4), these reduced rates will likely not result in a near-term decline in lung cancer mortality. With a current base of over 90 million current or former smokers, lung cancer will continue to pose a major challenge for the foreseeable future (5). Worldwide, lung cancer rates are expected to rise in the coming years due to the increased prevalence of smoking in developing countries (5). Other significant risk factors for lung cancer include exposure to radon gases and occupational exposures such as asbestos and arsenic. Recent advances in our understanding of tumor biology have also helped us understand more about predictive and prognostic factors in lung cancer. DNA repair pathways such as ERCC-1 (6) EGFR and ALK mutational status (7), in addition to several other emerging molecular targets will likely play a pivotal role in the prognosis and treatment decisions in lung cancer in the years to come.
Recent efforts to institute a lung cancer screening program have had some success. The lung cancer screening trial, published in 2011, showed that in current or heavy previous smokers between 55-74, conducted prospectively, that there was a 20% reduction in mortality for those screened with low dose helical CT scan vs. those with chest X-ray (8). The American Cancer Society and the American Thoracic Society have issued screening recommendations. Recently, the United States Preventive Task Force has also voiced their support and recommended screening for individuals between 55 and 80 who currently smoke or have a 30 pack year smoking history who have quit within the previous 15 years. Unfortunately, the Task Force also limits its recommendation for screening to those with the willingness or ability to have curative lung surgery. This may exclude patients that may benefit from the other emerging treatment modality for early stage lung cancer, stereotactic body radiotherapy (SBRT). Despite this, there is hope that implementation of screening methods in the coming years may increase the percentage of lung cancer diagnosed in earlier, more treatable stages, and may help further develop SBRT as a reasonable treatment approach.
Treatment of early stage lung cancer
The standard of care for definitive treatment of early stage lung cancer continues to be surgical resection with lobectomy or pneumonectomy. Several large trials have been reported, showing five year overall survival for patients with stage IA and IB disease of 71.25% and 57%, respectively (9-11). Lesser surgical resections, to include wedge resections, have shown worse local control and a trend toward worse overall survival (12). Less invasive techniques continue to be evaluated, but the current standard surgical approach remains thoracotomy (11).
Patients who either decline surgery or are considered unacceptable surgical candidates due to morbidity or mortality concerns are generally offered treatment with radiation therapy alone. Using traditional methods to deliver radiation therapy, results for these patients have been inferior to those for patients treated with surgical resection. There are several potential explanations for these worse outcomes. First, there is an obvious selection bias. Patients treated with radiation represent a high-risk group of patients with more medical comorbidities and resultant worse performance status than those patients offered surgical treatment. Secondly, the patients treated with surgery alone undergo pathological staging as opposed to the clinical-only staging of the patients treated with radiation alone. Undoubtedly some patients with clinically early stage disease would have been found to have more advanced disease on formal pathologic analysis (13). This is especially true for studies before the more modern CT or even PET/CT era. Still, it must be remembered that reported results for patients treated with radiation alone have shown worse local control as well as survival than those seen with resection. Reported 5-year survival in patients treated with traditional radiation treatment range from 6-32%. Local-only failure has been reported in 39-55% of patients (14-17).
The dose of radiation used in treating these patients remained stable for many years. Based on the increased radiologic control seen with increasing dose in RTOG 7301, the standard radiation dose has been 60 Gy. About 65% of the patients treated with at least 60 Gy were found to have local control (18). However, these results likely overstate the true rate of local control with this radiation dose, given their reliance on clinical evidence of local failure. Another series evaluated local control more rigorously (including bronchoscopy) and found local control after 65 Gy given without or with combination chemotherapy of 17% and 15%, respectively (19).
Multiple strategies for improving outcomes with radiation alone have been employed. The most obvious approach has been with dose escalation of conventionally-fractionated radiation. One cooperative group trial reported outcomes with doses of up to 83.8 Gy for patients with V20 of <25% using a fraction size of 2.15 Gy. This phase I/II trial demonstrated acceptable toxicities but did not show a dose response across the range of 70.9-83.8 Gy (20). Unfortunately, a large multi-institutional randomized controlled study comparing 60-74 Gy in locally advanced patients, also failed to show any survival benefit (21). However, other single institution studies have been reported, with a series from Michigan delivering doses as high as >100 Gy with acceptable toxicities (22). A series from Memorial Sloan Kettering Cancer Center (MSKCC) showed a local control advantage with increased dose, with local control at 2 years of 14% for stage I-II patients receiving <80 Gy and 88% for like-stage patients receiving >80 Gy. This series also reported a doubling in medial survival for patients treated with >80 Gy (23).
Another important treatment approach that has been affirmed in the above studies, mostly in an effort to minimize toxicity, has been to avoid elective nodal irradiation (ENI), and instead target only areas of gross disease. The results of these and other series offer strong arguments against the elective treatment of at-risk areas of lymph drainage without evidence of malignant involvement (24). An early report from MSKCC showed a crude rate of elective nodal failure in 171 patients treated without ENI of 6.4% (25); the 2-year actuarial rate of failure was 9%. In RTOG 9311 and the series from Michigan, no ENI was used and the resultant failure in these areas was less than 10% (20) and zero (22), respectively.
Rationale for stereotactic radiotherapy
Standard fractionated radiation treatment is usually given over a course of 6 to 7 weeks, at 1.8-2.0 Gy per fraction. Changes to this schedule, such as increasing dose with a longer course of conventional fractionation has had mixed success of improving local control. One important consideration in dose escalation involves the increase amount of time required to give larger overall doses. Any advantage gained by an increase in dose must be balanced against a detriment seen with prolongation of overall treatment time. There are radiobiologic studies to support this dilemma. One modeling study suggested that conventionally-fractionated doses of >100 Gy would be required to provide 90% local control at 30 months. To achieve this dose would require 10 weeks, instead of the usual 6-7 weeks, of treatment with conventional fractionation (26).
Another problem is that treatment interruptions are more frequent with increasing radiation dose. Analysis of three RTOG trials from the 1980s showed a detriment in overall survival, particularly seen in patients with otherwise favorable prognostic factors (27). Another pooled-analysis of three RTOG trials from the 1990s evaluated treatment time as a continuous variable. Of 474 total patients analyzed, 18% (n=87) patients had prolongation of treatment time of more than five days. Multivariate analysis showed that each day of prolongation translated to a 2% increase in risk of death (28). Hyperfractionation, or more than one daily fraction spaced at least 6 hours apart, is one approach to try and avoid prolonged treatment delivery times. However, once again, results have thus far have been mixed (29-31).
SBRT, in contrast, allows for a high dose to be delivered in one or only a few treatment fractions. This is possible based off technical advances with the use of intensity modulation using, for example, multi-leaf collimation. There are several potential advantages gained with this treatment method. These include prevention of accelerated repopulation, the ability to treat with BEDs in excess of 100, and treating to a point on the steepest portion of the cell survival curve (26,32,33). There is also the added convenience for the patient of requiring few trips for treatment. Using SBRT techniques to treat malignancy is becoming the object of study in several areas of the body. Tumors of the liver, pancreas, cervix and head and neck have growing bodies of evidence suggesting the role of SBRT in management in certain situations. In the brain, radiosurgery (SRS, single fraction stereotactic radiation therapy) has a well-established role in the treatment of both benign and malignant tumors. Intracranial targets appear particularly well-suited to radiosurgical approaches. Reasons for this include, first, bony anatomy is a reliable surrogate for target position (34) and second, reliable immobilization can help eliminate internal intrafraction target motion (35). Contrarily, thoracic targets are neither well-localized by external anatomy nor are they static (36,37). These represent the two largest treatment-delivery related hurdles to delivering SBRT to targets within the lungs, and several techniques and devices have been developed to overcome these problems.
Motion management
Historically, achievement of reproducible patient positioning and thus accuracy, was best accomplished with the use of a rigid external frame. This is, in fact, the essence of stereotaxy, defined as the use of position and movement through space. True stereotactic treatment relies on the generation and use of an external 3-dimensional (3D) coordinate system; any point within this coordinate system can be positionally described by its relation to the external framework. However, the use of rigid external frames has its own limitations. They tend to be cumbersome, large and often require sedation or other methods of pain control in order to be tolerated by patients. In the last two decades, we have seen the emergence of imaging coupled with the treatment machine with 3D techniques, so called “on board imaging”. One example of this imaging is Cone Beam Computed Tomography (CBCT). Producing a 3D CT scan in real time can achieve comparable localization to stereotaxy. The addition of placement of implanted fiducial markers, discussed in more detail below, may offer an even more robust and reproducible framework for accuracy of tumor targeting.
Intrafraction motion management presents another impediment to lung SBRT. Once again, recent technical advancements are helping overcome this obstacle. Roughly, these technical advancements can be divided into two groups. The first group allows the target to move freely relative to the treatment beams, but accounts for this motion with either geometric or dosimetric considerations. The most basic technique employs the concept of an integrated tumor volume (ITV). Early SBRT studies of the lung utilized this method. It includes the addition of margins based on population averages. For example, looking at typical inspiratory and expiratory position changes, a plan may involve arbitrarily adding 2 cm in all directions in all cases. In most cases, this population-derived margin is bigger than required and results in treatment of a larger volume of normal lung. The addition of patient-specific margins can be achieved by using fluoroscopic monitoring of target motion throughout the respiratory cycle. An emerging technique, most commonly described as 4D CT scanning, can scan throughout physiologic movement and place position into specified bins of location for each phase of the respiratory cycle. Less technically, one may obtaining both end-inspiratory and end-expiratory imaging and creating a planning target volume that encompasses both extremes.
Strategies in the second group are aimed at maintaining the target position relative to the treatment beams throughout treatment. Within this group are techniques aimed at reducing target motion. Some of these include abdominal compression, deep inspiratory breath hold (DIBH), or forced shallow breathing. Also within this group are techniques which allow for target motion but adjust treatment delivery to maintain constancy between target and treatment beams. These include respiratory gating, beam tracking, or couch-based motion compensation. Data are published that support these techniques. For example, in patients treated for liver metastases, application of abdominal pressure has been found to reduce excursion of the diaphragm to 7 mm (38). DIBH has been shown to increase total lung volume, decrease lung mass within expansion margins, and provide relatively reproducible target displacements (39). Another series found DIBH to be reproducible and reported a potential 30% decrease in the V25 by reducing required target margins (40). Reducing margins from 2.5 to 0.5 cm was shown to decrease by 66% the amount of normal lung in the treatment volume.
Respiratory gating requires use of a modern CT simulation machine able to obtain data in four dimensions, dividing the respiratory cycle into typically 10 bins. This allows target visualization throughout the respiratory cycle and selection of an appropriate range of phases for treatment planning and delivery. At the time of treatment, the patient’s respirations are monitored via a reflective box placed on the midsection and the treatment beam is selectively turned off and on at the predetermined phases of respiration (41).
Implanted fiducial markers can be used both for patient positioning as well as target tracking during treatment delivery (42). Using an implanted gold marker seed, one series found that real-time tracking with free breathing could be utilized. In this series, the treatment beam was gated based on the position of the gold marker, successfully reducing the target motion during beam delivery to within 5.3 mm (43).
Beam tracking is another technique. Systems using a dynamic robotic arm and traditional linear accelerators with multi-leaf collimators are both able to track target motion through the sliding collimator leaves (44,45). Finally, there is interest in couch-based motion management, in which the treatment couch motion is equal and opposite to target motion; this technique may be able to maintain consistency in the beam’s eye view during treatment delivery (46).
At our institution, we routinely use SBRT for treatment of early stage lung cancer. At the time of simulation, 4D CT scans are obtained to delineate an ITV. Tight margins in the range of 5-7 mm are employed around the ITV to limit dose to normal lung tissue and other critical organs near the target. The use of a gating technique is individualized based on quantitative tumor motion. We use an algorithm that employs respiratory gating in patients with more than 5 mm of motion on 4D CT in those with non-apical tumors. The gating window is determined based on the pattern of tumor motion during different phases of respiratory cycle. A typical plan for SBRT of a primary lung cancer is shown in Figure 1. Image guided radiation therapy is delivered by use of CBCT to localize the target and make appropriate shifts prior to each treatment. Respirtory motion is monitored using the RPM system (Varian Medical Systems, Inc, Palo Alto, CA). Other systems, such as VisionRT (Varian Medical Systems, Inc) are currently under development as alternatives to the RPM system.
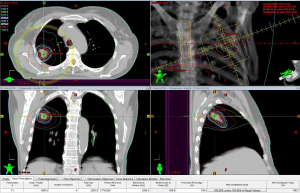
Early clinical experience with SBRT
Following early pioneering reports on stereotactic treatments primarily of liver and lung tumors (47,48), a phase I study by Timmerman et al. reported the results of 37 medically inoperable patients treated with SRT. The maximum tumor size was 7 cm, and all patients were treated using a rigid immobilization frame along with abdominal pressure. Gross tumor volume (GTV) was expanded by 0.5 cm radially and 1.0 cm cranio-caudally to create PTV. Three fractions were delivered, starting with 8 Gy to the 80% isodose line with increases up to 22 Gy per fraction. The maximum tolerated dose (MTD) was not reached in this series. With median follow up of 15.2 months, there were two instances of acute grade three lung toxicity and no instances of late lung toxicity reported. The overall response rate was 87%. There were six patients with local failure, two of which also failed distantly; no patient treated with fraction sizes of >18 Gy experienced local failure (49). This experience was subsequently updated, now including 47 total patients. MTD had still not been reached for patients with T1 tumors, however three of five patients with T2 tumors larger than 5 cm experienced grade three or higher toxicity at the 72 Gy dose level. The MTD for these patients was therefore 66 Gy (22 Gy ×3 fractions). Of ten local failures, nine were seen in patients treated with <16 Gy per fraction (50).
Based on these encouraging results, a phase II investigation was undertaken and published in 2006. This series included 70 medically inoperable patients with stage T1N0 (n=35) or T2N0 (n=35) non-small cell lung cancer (NSCLC) treated with 3 fractions of 20 or 22 Gy, respectively, in the same manner as above. With median follow up of 17.5 months, two year local control was 95%. Unfortunately, eight patients had experienced grade 3-4 toxicity; there were also a total of six grade 5 toxicities, occurring from 0.6 to 19.5 months post-treatment. Four of these were due to pneumonia, while the patient who experienced death at 19.5 months died as a result of massive hemoptysis. The authors advised caution when treating patients with centrally located tumors due to the observed increase in toxicity (51). Reports from other institutions have supported the finding of increased toxicity for central tumors. RTOG 0813 is a phase I/II study that is looking specifically at centrally located tumors and is looking at dose escalation of 5 fractions delivered over 1.5-2 weeks. The study started at 50 Gy in 5 fractions and had gotten to 12 Gy per fraction, but preliminary reports are showing some increased toxicity at that level. Final results have not been reported, but will most likely recommend doses in the range of 10-11 Gy per fraction with total doses of 50-55 Gy.
RTOG 0236 was designed to mirror the single-institution phase II study at the University of Florida detailed above. Patients enrolled on this phase II study were to receive a total of 60 Gy in three 20 Gy fractions. Patients with tumors of the proximal bronchial tree were excluded from this experience. While a 3D coordinate system was required, implanted fiducial markers were accepted as a replacement for external fixation. Treatment margins were as above. They found 3-year tumor control of 98%, 3-year local control of 91% and 3-year loco-regional control of 87%. Median overall survival was 48 months and there was a 17% grade 3-4 toxicity rate with no grade 5 toxicity (52).
Reports from overseas have also been encouraging. A large retrospective Japanese series included a total of 275 patients with stage T1N0 (n=164) or T2N0 (n=93) lung cancer were treated with a range of doses, from 18-75 Gy given in 1-22 treatment fractions. This series reported excellent outcomes, particularly those who received a biological equivalent dose (BED) of more than 100. Local failure occurred in 8.1% of those patients treated to a BED of >100 Gy, and the 5-year overall survival of operable patients in this high-dose group was 71%. This compares to a 5-year overall survival of 30% for operable patients treated to lower doses (53).
Based off these findings, the Japan Clinical Oncology Group opened a phase II trial of operable patients using a dose fractionation scheme of 48 Gy in 4 fractions. They enrolled 64 patients and showed an overall survival of 76% with 6.1% grade 3 toxicity rate with no grade 4 or 5 toxicity. In parallel, the RTOG launched 0618 and closed to accrual in May 2010 meeting their goal of 33 patients. They used 18 Gy in three fractions and limited the study to peripheral lesions. They reported their findings at the 2013 ASCO annual meeting and reported a 16% grade three toxicity rate with no grade four or five toxicity. They showed a 2-year tumor control rate of 92.3%, a regional control rate of 88.3% and a distant failure rate of 15.4%. Two-year overall survival was 84.4%. An ASOSOG/RTOG joint trial attempted a Phase III study direction comparing sublobar resection with SBRT in high risk patients with stage I NSCLC. Unfortunately, after 2 years, the study had only accrued 10% of its target of 422 patients and closed in May 2013.
In Germany, a parallel experience using single fraction treatment was reported by Fritz et al. Maximal tumor size was <10 cm and central lesions were excluded. Thirty-seven of 40 treated patients were medically inoperable. A rigid stereotactic frame was used, but abdominal compression was not. Patients received CT scans at end-inhalation, end-expiration, and mid-cycle. Expansions to PTV were larger, at 15 mm cranio-caudally and 10 mm radially. Dose to the isocenter was 30 Gy, with at least 80% coverage of the PTV. All patients responded to treatment, with 47.5% showing radiographic complete response (CR). There were a total of three local recurrences, giving an actuarial local control of 81% at 3 years. Asymptomatic radiation pneumonitis was seen in 75%, and transient pleural effusions in 25% of patients. The only reported grade four toxicities were rib fracture in 5% of patients (54). The RTOG has opened a phase II trial comparing the German vs. the Japanese fractionation scheme in RTOG 0915. They are looking at stage I peripheral tumors who will receive either 34 Gy in a single fraction (arm 1) or 48 Gy in four once daily consecutive fractions (arm 2). The primary objective was to assess toxicity of the two fractionation schemes. Accruing 94 patients they reported in 2013 that adverse events were similar (P=0.337) as was local control (97.1% and 97.6%, respectively) and concluded that 34 Gy in 1 fractions would be used as the experimental arm in a planned phase III trial (55).
Future directions of study
One common scenario in the work up of a newly identified suspicious lung lesion is the lack of a pathology-confirming biopsy. In patients with advanced obstructive and/or restrictive lung disease the morbidity of biopsy may outweigh the need for tumor confirmation. Bradley et al. reported a series of 91 patients treated with SBRT, of which 24% were treated without biopsy-proven NSCLC (56). They observed no difference in local control between patients with vs. without biopsy proven NSCLC. Also, Verstegen et al. compared patients with vs. without biopsy proven NSCLC treated with SBRT and observed no difference in local control or overall survival (57). At our institution, we recently reported on a series of 55 patients treated with SBRT, 23 without pathologic tumor confirmation. With 24 months of median follow-up, within the group without tumor verification we found an 8.7% local failure rate and a 12-month overall survival of 83%. On Kaplan-Meier analysis there was no significant difference in overall survival between the patients with and without pathologic confirmation of malignancy (P=0.27) (58). Obviously, larger prospective studies will need to be accomplished to more accurately show the validity of treatment in patients without confirmed NSCLC. Likewise, SBRT appears to be a safe and effective alternative in elderly patients over 80 years old (59).
Another scenario where SBRT may play an important role is in the identification of multiple synchronous or metachronous primary lung cancers (MPLC). There is ample evidence that surgical resection remains the primary modality of treatment of these patients (60-62), but often, these patients are not surgical candidates. Given the high relative risk of smokers that will be screened based on recent US task force recommendations, multiple synchronous tumors may increase from its current rate of 1% to 4% of current lung cancer diagnoses (63). We recently reported on a series of 18 patients with 36 separate MPLC lesions. A total of 16 were not surgical candidates and 2 had refused surgery. At a median follow up of 20 months we observed local control of 81.5% with overall survival at 2 years of 62%. Grade 3 pneumonitis occurred in 17% of patients, all successfully treated with steroid therapy (64). Figure 2 is an example of a plan to treat multiple primaries.
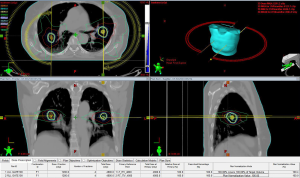
The role of SBRT to oligometastatic disease in the lung remains another area of active research. To date, the literature consists of retrospective, single institutional reviews. Results have been encouraging with good local control and 2- to 3-year survival (65-68).
Conclusions
SBRT is being widely adopted as definitive treatment for patients with inoperable early stage NSCLC. While more technically challenging, techniques to compensate for motion management and tumor identification are allowing more accurate and tighter treatment fields. Specific dosing and fractionation schemes are not standardized, but some caution should be used for centrally located tumors. Current studies continue to evaluate 1-, 3-, 4- and 5-fraction schedules. Safety, local control and even impact on survival have been encouraging. There are emerging studies on the role of SBRT in operable patients and an interest in comparing surgical resection with this novel treatment. Whether these comparisons will reach target accrual goals remains to be seen. Finally, SBRT may have unique benefits in treating patients unable to undergo biopsy, and in the setting of multifocal, recurrent and oligometastatic disease.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Sandra Vermuelen, Kevin T. Murphy, Huan Giap) for the series “SBRT/SRS in Radiation Research” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2014.07.02). The series “SBRT/SRS in Radiation Research” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- American Cancer Society. Cancer Facts & Figures 2014. Atlanta: American Cancer Society; 2014.
- Bach PB, Jett JR, Pastorino U, et al. Computed tomography screening and lung cancer outcomes. JAMA 2007;297:953-61. [PubMed]
- International Early Lung Cancer Action Program Investigators. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med 2006;355:1763-71. [PubMed]
- Pierce JP, Messer K, White MM, et al. Prevalence of heavy smoking in California and the United States, 1965-2007. JAMA 2011;305:1106-12. [PubMed]
- Jemal A, Simard EP, Dorell C, et al. Annual Report to the Nation on the Status of Cancer, 1975-2009, featuring the burden and trends in human papillomavirus(HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst 2013;105:175-201. [PubMed]
- Olaussen KA, Dunant A, Fouret P, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med 2006;355:983-91. [PubMed]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [PubMed]
- National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [PubMed]
- Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest 1997;111:1710-7. [PubMed]
- Naruke T, Goya T, Tsuchiya R, et al. Prognosis and survival in resected lung carcinoma based on the new international staging system. J Thorac Cardiovasc Surg 1988;96:440-7. [PubMed]
- Smythe WRAmerican College of Chest Physicians. Treatment of stage I non-small cell lung carcinoma. Chest 2003;123:181S-187S. [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [PubMed]
- Kopp R, Weidenhagen R, Reinmiedl J, et al. Outcome following lung resections for pT1 non-small cell lung cancer. Eur J Surg Oncol 2006;32:329-34. [PubMed]
- Dosoretz DE, Galmarini D, Rubenstein JH, et al. Local control in medically inoperable lung cancer: an analysis of its importance in outcome and factors determining the probability of tumor eradication. Int J Radiat Oncol Biol Phys 1993;27:507-16. [PubMed]
- Dosoretz DE, Katin MJ, Blitzer PH, et al. Medically Inoperable Lung Carcinoma: The Role of Radiation Therapy. Semin Radiat Oncol 1996;6:98-104. [PubMed]
- Sibley GS. Radiotherapy for patients with medically inoperable Stage I nonsmall cell lung carcinoma: smaller volumes and higher doses--a review. Cancer 1998;82:433-8. [PubMed]
- Slotman BJ, Antonisse IE, Njo KH. Limited field irradiation in early stage (T1-2N0) non-small cell lung cancer. Radiother Oncol 1996;41:41-4. [PubMed]
- Perez CA, Pajak TF, Rubin P, et al. Long-term observations of the patterns of failure in patients with unresectable non-oat cell carcinoma of the lung treated with definitive radiotherapy. Report by the Radiation Therapy Oncology Group. Cancer 1987;59:1874-81. [PubMed]
- Le Chevalier T, Arriagada R, Quoix E, et al. Radiotherapy alone versus combined chemotherapy and radiotherapy in unresectable non-small cell lung carcinoma. Lung Cancer 1994;10:S239-44. [PubMed]
- Bradley J, Graham MV, Winter K, et al. Toxicity and outcome results of RTOG 9311: a phase I-II dose-escalation study using three-dimensional conformal radiotherapy in patients with inoperable non-small-cell lung carcinoma. Int J Radiat Oncol Biol Phys 2005;61:318-28. [PubMed]
- PL03. 05 An intergroup randomized phase III comparison of standard-dose (60 Gy) vs high-dose (74 Gy) chemoradiotherapy (CRT) +/- cetuximab (cetux) for stage III non-small cell lung cancer (NSCLC): results on cetux from RTOG 0617. Clin Adv Hematol Oncol 2014;12:2-4. [PubMed]
- Hayman JA, Martel MK, Ten Haken RK, et al. Dose escalation in non-small-cell lung cancer using three-dimensional conformal radiation therapy: update of a phase I trial. J Clin Oncol 2001;19:127-36. [PubMed]
- Rosenzweig KE, Fox JL, Yorke E, et al. Results of a phase I dose-escalation study using three-dimensional conformal radiotherapy in the treatment of inoperable nonsmall cell lung carcinoma. Cancer 2005;103:2118-27. [PubMed]
- Emami B, Mirkovic N, Scott C, et al. The impact of regional nodal radiotherapy (dose/volume) on regional progression and survival in unresectable non-small cell lung cancer: an analysis of RTOG data. Lung Cancer 2003;41:207-14. [PubMed]
- Rosenzweig KE, Sim SE, Mychalczak B, et al. Elective nodal irradiation in the treatment of non-small-cell lung cancer with three-dimensional conformal radiation therapy. Int J Radiat Oncol Biol Phys 2001;50:681-5. [PubMed]
- Martel MK, Ten Haken RK, Hazuka MB, et al. Estimation of tumor control probability model parameters from 3-D dose distributions of non-small cell lung cancer patients. Lung Cancer 1999;24:31-7. [PubMed]
- Cox JD, Pajak TF, Asbell S, et al. Interruptions of high-dose radiation therapy decrease long-term survival of favorable patients with unresectable non-small cell carcinoma of the lung: analysis of 1244 cases from 3 Radiation Therapy Oncology Group (RTOG) trials. Int J Radiat Oncol Biol Phys 1993;27:493-8. [PubMed]
- Machtay M, Hsu C, Komaki R, et al. Effect of overall treatment time on outcomes after concurrent chemoradiation for locally advanced non-small-cell lung carcinoma: analysis of the Radiation Therapy Oncology Group (RTOG) experience. Int J Radiat Oncol Biol Phys 2005;63:667-71. [PubMed]
- Machtay M, Washam C, Devine P. Pilot study of accelerated radiotherapy with concurrent chemotherapy for stage III non-small cell lung cancer. Semin Oncol 2005;32:S9-12. [PubMed]
- De Ruysscher D, Wanders R, van Haren E, et al. HI-CHART: a phase I/II study on the feasibility of high-dose continuous hyperfractionated accelerated radiotherapy in patients with inoperable non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2008;71:132-8. [PubMed]
- Saunders M, Dische S, Barrett A, et al. Continuous, hyperfractionated, accelerated radiotherapy (CHART) versus conventional radiotherapy in non-small cell lung cancer: mature data from the randomised multicentre trial. CHART Steering committee. Radiother Oncol 1999;52:137-48. [PubMed]
- Brodin O, Lennartsson L, Nilsson S. Single-dose and fractionated irradiation of four human lung cancer cell lines in vitro. Acta Oncol 1991;30:967-74. [PubMed]
- Carmichael J, Degraff WG, Gamson J, et al. Radiation sensitivity of human lung cancer cell lines. Eur J Cancer Clin Oncol 1989;25:527-34. [PubMed]
- Guckenberger M, Baier K, Guenther I, et al. Reliability of the bony anatomy in image-guided stereotactic radiotherapy of brain metastases. Int J Radiat Oncol Biol Phys 2007;69:294-301. [PubMed]
- Hoogeman MS, Nuyttens JJ, Levendag PC, et al. Time dependence of intrafraction patient motion assessed by repeat stereoscopic imaging. Int J Radiat Oncol Biol Phys 2008;70:609-18. [PubMed]
- Wulf J, Hädinger U, Oppitz U, et al. Stereotactic radiotherapy of extracranial targets: CT-simulation and accuracy of treatment in the stereotactic body frame. Radiother Oncol 2000;57:225-36. [PubMed]
- Purdie TG, Bissonnette JP, Franks K, et al. Cone-beam computed tomography for on-line image guidance of lung stereotactic radiotherapy: localization, verification, and intrafraction tumor position. Int J Radiat Oncol Biol Phys 2007;68:243-52. [PubMed]
- Herfarth KK, Debus J, Lohr F, et al. Extracranial stereotactic radiation therapy: set-up accuracy of patients treated for liver metastases. Int J Radiat Oncol Biol Phys 2000;46:329-35. [PubMed]
- Cheung PC, Sixel KE, Tirona R, et al. Reproducibility of lung tumor position and reduction of lung mass within the planning target volume using active breathing control (ABC). Int J Radiat Oncol Biol Phys 2003;57:1437-42. [PubMed]
- Hanley J, Debois MM, Mah D, et al. Deep inspiration breath-hold technique for lung tumors: the potential value of target immobilization and reduced lung density in dose escalation. Int J Radiat Oncol Biol Phys 1999;45:603-11. [PubMed]
- Jiang SB. Technical aspects of image-guided respiration-gated radiation therapy. Med Dosim 2006;31:141-51. [PubMed]
- Wurm RE, Gum F, Erbel S, et al. Image guided respiratory gated hypofractionated Stereotactic Body Radiation Therapy (H-SBRT) for liver and lung tumors: Initial experience Acta Oncol 2006;45:881-9. [PubMed]
- Shimizu S, Shirato H, Ogura S, et al. Detection of lung tumor movement in real-time tumor-tracking radiotherapy. Int J Radiat Oncol Biol Phys 2001;51:304-10. [PubMed]
- Murphy MJ. Tracking moving organs in real time. Semin Radiat Oncol 2004;14:91-100. [PubMed]
- Rangaraj D, Papiez L. Synchronized delivery of DMLC intensity modulated radiation therapy for stationary and moving targets. Med Phys 2005;32:1802-17. [PubMed]
- D’Souza WD, Naqvi SA, Yu CX. Real-time intra-fraction-motion tracking using the treatment couch: a feasibility study. Phys Med Biol 2005;50:4021-33. [PubMed]
- Blomgren H, Lax I, Näslund I, et al. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol 1995;34:861-70. [PubMed]
- Nádvorník P, Kolarík J, Bolf J. First model of whole body computerized stereotactic device. Acta Univ Palacki Olomuc Fac Med 1989;121:149-52. [PubMed]
- Timmerman R, Papiez L, McGarry R, et al. Extracranial stereotactic radioablation: results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest 2003;124:1946-55. [PubMed]
- McGarry RC, Papiez L, Williams M, et al. Stereotactic body radiation therapy of early-stage non-small-cell lung carcinoma: phase I study. Int J Radiat Oncol Biol Phys 2005;63:1010-5. [PubMed]
- Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006;24:4833-9. [PubMed]
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [PubMed]
- Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol 2007;2:S94-100. [PubMed]
- Fritz P, Kraus HJ, Blaschke T, et al. Stereotactic, high single-dose irradiation of stage I non-small cell lung cancer (NSCLC) using four-dimensional CT scans for treatment planning. Lung Cancer 2008;60:193-9. [PubMed]
- Videtic G, Hu C, Singh A, et al. Radiation Therapy Oncology Group (RTOG) Protocol 0915: A Randomized Phase 2 Study Comparing 2 Stereotactic Body Radiation Therapy (SBRT) Schedules for Medically Inoperable Patients With Stage I Peripheral Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2013;87:S3.
- Bradley JD, El Naqa I, Drzymala RE, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung cancer: the pattern of failure is distant. Int J Radiat Oncol Biol Phys 2010;77:1146-50. [PubMed]
- Verstegen NE, Lagerwaard FJ, Haasbeek CJ, et al. Outcomes of stereotactic ablative radiotherapy following a clinical diagnosis of stage I NSCLC: comparison with a contemporaneous cohort with pathologically proven disease. Radiother Oncol 2011;101:250-4. [PubMed]
- Haidar YM, Rahn DA 3rd, Nath S, et al. Comparison of outcomes following stereotactic body radiotherapy for non-small cell lung cancer in patients with and without pathological confirmation. Ther Adv Respir Dis 2014;8:3-12. [PubMed]
- Sandhu AP, Lau SK, Rahn D, et al. Stereotactic body radiation therapy in octogenarians with stage I lung cancer. Clin Lung Cancer 2014;15:131-5. [PubMed]
- Carretta A, Ciriaco P, Melloni G, et al. Surgical treatment of multiple primary adenocarcinomas of the lung. Thorac Cardiovasc Surg 2009;57:30-4. [PubMed]
- Chang YL, Wu CT, Lee YC. Surgical treatment of synchronous multiple primary lung cancers: experience of 92 patients. J Thorac Cardiovasc Surg 2007;134:630-7. [PubMed]
- Rea F, Zuin A, Callegaro D, et al. Surgical results for multiple primary lung cancers. Eur J Cardiothorac Surg 2001;20:489-95. [PubMed]
- Verhagen AF, Tavilla G, van de Wal HJ, et al. Multiple primary lung cancers. Thorac Cardiovasc Surg 1994;42:40-4. [PubMed]
- Rahn D, Thakur S, Makani S, et al. Stereotactic body radiation therapy (SBRT) for multiple primary lung cancers (MPLC): a review and case series. Journal of Radiosurgery & SBRT 2013;2:135.
- Navarria P, Ascolese AM, Tomatis S, et al. Stereotactic body radiotherapy (sbrt) in lung oligometastatic patients: role of local treatments. Radiat Oncol 2014;9:91. [PubMed]
- Singh D, Chen Y, Hare MZ, et al. Local control rates with five-fraction stereotactic body radiotherapy for oligometastatic cancer to the lung. J Thorac Dis 2014;6:369-74. [PubMed]
- Filippi AR, Badellino S, Guarneri A, et al. Outcomes of single fraction stereotactic ablative radiotherapy for lung metastases. Technol Cancer Res Treat 2014;13:37-45. [PubMed]
- Baschnagel AM, Mangona VS, Robertson JM, et al. Lung metastases treated with image-guided stereotactic body radiation therapy. Clin Oncol (R Coll Radiol) 2013;25:236-41. [PubMed]


