
A review of cerebral arteriovenous malformations and treatment with stereotactic radiosurgery
Cerebral arteriovenous malformations (AVMs) have a complicated and contentious history. AVMs are currently defined as congenital vascular malformations that consistent of feeding arteries and draining veins with a network of vessels called a nidus that lack an intervening capillary bed (1). Other terms have been used to describe these lesions, including angioma arteriale racemosum (2) varix aneurysmaticus (3) arteriovenous angiomas, and proliferative capillaropathy (4). Some even consider cerebral AVMs fistulized cerebral venous angiomas (4-6).
Definition
AVMs can be located anywhere and can vary widely in size. Distal arterial branches are more commonly involved and predominately the MCA and hemispheric convexities (7). They are generally solitary lesions that are thought to be congenital and occur sporadically. Multiple lesions typically are syndromic with cutaneous or extracranial syndromes such as Rendu-Osler-Weber disease, Wyburn-Mason syndrome, and Sturge-Weber syndrome (8-11).
Cerebral AVMs are often pyramid-shaped with a base adjacent to cortex with the vertex projecting internally towards the ventricles. Blood flow is higher through AVMs than normal brain parenchyma. There may be single or multiple feeding arteries, which are often times tortuous and branching. They can vary in caliber and wall thickness. Chronic high blood flow in arterial feeders may cause stenotic or dilated vessel changes with endothelial thickening, intimal hyperplasia, abnormal or absence media and elastic lamina (12-15). The high flow and low resistance shunting in AVMs may recruit collateral supply from surrounding territories, called angiomatous change. The arterial feeders terminate in the nidus but some may go on to supply blood to distal parenchyma. Associated aneurysms occur in 10-50%, with multiple not being unusual (16-19).
The nidus is defined as the convergence of the feeding arteries and from which enlarged draining veins emerge. The nidus is typically tightly organized and displaces normal brain parenchyma. There may be normal intervening parenchyma with loosely defined niduses (20). There can be a diversity of shunting within niduses with simple fistulas to complex plexuses. Accurate and consistent measurements are difficult. Nidal vessels may have hypertrophic media, which can blur the line between artery and vein.
The drainage veins are one or more dilated veins that form deep within the nidus and reach the superficial and/or deep venous system. Deep draining veins being defined as internal cerebral veins, basal veins, or precental cerebellar veins. The high transmural pressures from the arterial system are transmitted to the compliant venous system, which may cause venous hypertension. These draining veins are often abnormal due to the hemodynamic stresses causing stenosis, ectasia, and varix formations (21,22).
Pathogenesis
AVMs may develop from a disruption of normal vascular morphogenesis during fetal development, possibly during the lissencephalic state. A persistent artery to artery connection at that time and many AVMs are located in the arterial borderzone territories (23-25). Vascular development within the brain typically occurs in two stages: vasculogenesis and angiogenesis. Initially endothelial cells come from angioblasts and form a primary vascular plexus. Angiogenesis than occurs with remodeling and reorganization of the primary vascular plexus by complex protein signaling pathways (26). Unfortunately the particular pathogenesis of AVMs is still unknown. Some suggest AVMs may be a persistent congenital vascular plexus without remodeling since arterialized veins are similar to the embryonal pattern (27-29). While others suggest that AVMs are dynamic and are a product of proliferative capillaropathy. This may occur due to disturbances in venous drainage and venous anomalies such as venous occlusion, stenosis, or agenesis (30-34).
Many cellular and molecular studies have been conducted on AVMs. Over 900 genes have been associated with AVMs (35). Over 300 are upregulated while almost 560 are downregulated (36). Vascular endothelial growth factors (VEGF) have been found to be expressed at high levels during embryonic development but are normally suppressed in the adult cerebral vasculature. VEGF-A has been found to be expressed by cells adjacent to AVMs while VEGF-C and D are highly expressed in AVMs with large niduses (37). Children with recurrent cerebral AVMs highly express VEGF in the endothelial layer and media of vessels in AVMs (38,39). ANG-2 expression is also upregulated in AVMs, is it 800% higher than expression rates. VEGF and ANG2 are key factors needed for tumor angiogenesis (40). ENG mutations have also been associated with a higher prevalence of cerebral AVMs (41,42). With the current cellular and biomolecular research, the pathogenesis of AVMs is still unclear.
Classifications
The most common classification system for AVMs is the Spetzler-Martin Grading Scale (SMG). It is classifies the degree of surgical difficulty and risk of surgical morbidity and mortality. It is a composite score based on nidal size, venous drainage, and eloquence of adjacent brain and ranges from 1-5. The score for nidal size ranges from 1-3, (1 point for a nidus <3 cm, two points for a nidus 3-6 cm, three points for a nidus >6 cm). If the AVM is located in the brainstem, thalamus, hypothalamus, cerebellar peduncles, or sensorimotor, language or primary visual cortex, 1 point is given, otherwise a no points are given. If there is any deep venous drainage, such as internal cerebral veins, basal veins, or precentral cerebellar veins, 1 point is given (43).
Epidemiology
The natural history and prevalence of AVMs is not completely understood. The prevalence of AVMs in the general population is 15-18 per 100,000 adults, which equates to approximately 0.05% of the population (44-47). They are incidentally found in 0.05% of brain MRIs (48). The current research has found that the incidence of symptomatic AVMs is between 1.1-1.84 per 100,000 per year (49,50). AVMs account for approximately 1.4-2% of all strokes and 9% in all primary ICHs, with approximately half of patients with AVMs presenting with an ICH (51-53).
Due to the heterogeneity of AVMs, annual hemorrhage risk may be as low as 0.9% per year for unruptured AVMs with superficial drainage, or as high as 34% per year for ruptured AVMs that are deeply seated, have associated aneurysms, and have deep venous drainage (54,55). Most use a rupture rate of approximately 2-4% (56,57). The crude annual fatality rate of 1-1.5%.
Clinical presentation
AVMs most often present with ICH, but may also present with unprovoked seizures, headaches and focal neurologic deficits.
Hemorrhage
Approximately 38-71% of patients with AVMs present with a hemorrhage with an incidence of first hemorrhage being 0.51 per 100,000 person years (46,47,52). Typically patients are between 20 and 40 years of age (49,58). The mean time interval between initial presentation and subsequent hemorrhage is 7.7 years (59).
Some studies have shown that increased resistance or high transnidal pressures may predispose an AVM to hemorrhage. Smaller AVMs are thought to be more prone to rupture than larger ones, possibly due to the higher resistance in smaller AVMs. Some report an inverse relationship between the size of the hematoma and that of the AVMs (60-68). Feeding artery pressures were higher in ruptured AVMs, 90.4% of MAP, compared to unruptured AVMs, 47% of MAP (69).
Once an AVM has hemorrhaged the risk of subsequent hemorrhage is elevated but will fall to baseline rates in subsequent years (53,55,70). The risk of subsequent hemorrhage range from 9.65% to 32.9% in the first year and falls to 3.67% to 11.3% in subsequent years (53,71).
An increased risk of hemorrhage is associated with AVMs that are deeply seated, infratentorial location, have associated aneurysms, and have deep venous drainage, typically periventricular, galenic, or cerebellar (72,73). AVMs with associated aneurysms have an overall risk of hemorrhage of 6.93% per year. The risk of hemorrhage is not altered by partially treating and AVM and remain until complete AVM obliteration is seen (53).
Each hemorrhage is associated with neurologic morbidity and mortality. Typically 20% to 30% have some neurologic morbidity and the mortality rates of hemorrhage range from 10% to 40% (50,56,59,66,67,74). In a Finish study, the mean age of death from an AVM hemorrhage was 44.4 years, which was significantly lower than patients dying from other causes in the study, 59.4 years. Yearly mortality rate was 1% per year and the rates of both hemorrhage and death remain constant during the study (59). At 11 days after rupture, the median Modified Rankin Scale (mRS) of 4 to 5 was 14%, and 11% have an NIHSS greater than 13. Age, gender, race, and size did not have a significant impact on outcome (75). In another study, at 16.2 months, 47% had no neurologic deficits, 37% were an mRS of 1 (independent), 13% were an mRS of 2 or 3 (moderately disabled), 3% were an mRS greater than 4 (severely disabled). In a Toronto study only 35% of patients had significant functional impairments with a GOS of 2 or 3 (76).
Seizures
Eighteen percent to forty percent of patients with AVMs present with a seizure (53,59,67). 30% of seizures were generalized tonic clonic seizures, while 10% were focal (58). AVMs that presented with seizure have demonstrated the shortest time to peak contrast density in the feeding vessels, possibly having higher flow in these vessels. They also tended to have shorter times for the arterial contrast density to decrease (77). The response rates with antiepileptic drugs are good, with the majority of patients controlled with medication (78).
Headaches
Five percent to fourteen percent of patients present with a headache without a hemorrhage (58). They do not have any distinctive characteristics. They can be unilateral, bilaterally, and can mimic migraines (79).
Focal neurologic deficits
Five percent to fifteen percent of patients present with persistent or progressive neurologic deficits (56,58,59,75). The pathophysiology of this phenomenon is unknown. Some suggest that deficits may be due to a vascular steal phenomenon due to high shunting through the AVM and resulting low cerebral blood flow in the surrounding brain. SPECT has shown decreased flow in the areas surrounding and distance to AVMs (80). On CT Perfusion studies, the local CBF is impaired area around AVMs and return to normal after excision, correlated with an improvement of deficits (81). The degree of vascular steal is inversely proportional to the resistance in the AVM itself. Neuropsychological testing also supports the idea of local and distance vascular steal (82). TCDs around medium and large AVMs provide no evidence of the steal phenomenon (83). And no relationships have been found between feeding artery pressures or flow velocities and focal neurologic deficits, putting into question the idea of steal phenomenon and hypoxia (84). Others suggest mass effect due to compressive venous dilatation on vulnerable white patter pathways may be the issue. The Columbia AVM databank has found an independent association of deficits with increasing age, female gender, deep location and venous ectasia (75,85).
Diagnosis
Cerebral angiography is the gold standard for the evaluation of AVM architecture. Initial imaging may be CT or MRI, which may be used for initial diagnosis.
CT
Patients may be initially evaluated with a CT. A spontaneous ICH, especially in younger patients or lobar location, unexplained ICH or SAH may go on to have additional studies performed, such as a CTA, which provides better vascular detail. Those without a hemorrhage, calcifications or hyperdense structures can represent draining veins, components of the nidus, or dilated arterial feeders may be seen on CT (86,87).
MRI/MRA
MRI and MRA may provide visualization of changes to the brain adjacent to the nidus such as perinidal or intranidal gliosis. Atrophy with focal dilatation of the ventricles, hemosiderin, hydrocephalus, ventricular compression of enlarged raining veins may be seen on MRI/MRA (87,88,89).
Functional MRI
Blood-oxygen-level dependent functional MRI may be helpful in providing information about eloquence in regards to structures in and around the AVM. It may be helpful in treatment planning (90,91). Unfortunately the neurovascular uncoupling phenomenon is a major limitation of functional MRIs for the evaluation of areas adjacent to AVMs. Dysplastic vessels may not demonstrate the appropriate activation of normal functioning neurons (92).
DSA
Cerebral angiography can visualize the morphology, location of the nidus, presence and location of associated aneurysms, arterial feeders and venous drainage patterns (87,88,93). Early DSA will reveal most AVMs. A normal study is often followed up with a delayed DSA in patients with a high suspicion of vascular malformations. Pretherapeutic planning often requires DSA. Endovascular embolization allows for targeting of specific feeding arteries and treatment of associated aneurysms. 3D angiography can aid in determination of radiation target for SRS planning as well.
Management
Observation/medical management
Ruptured AVMs have high rates of morbidity and mortality and have higher rates of subsequent hemorrhage, presenting a compelling justification for treatment. Unruptured AVMs are more challenging. In a large meta-analysis of observational studies, severe complications were observed in 5.1-7.4% and median obliteration rates of 13-96%. Case fatality decreased over time as did complication rates from SRS and embolization, most likely attributed to technological advances (94).
The ARUBA study was developed to help answer that question. The study was ended due to the increased rates of complications in the treatment arm compared to medical management. The study is being continued with extended follow up of both groups to fully evaluate the natural history (95,96). Observation and medical management is typically considered for those that are asymptomatic and who do not have a history of a hemorrhage. Symptoms such as seizures, headaches and hypertension are managed with medical therapy along with general medical care. Surveillance imaging is done but the interval and type of imaging is not well defined. Typically patients will have an MRI annually or biennially (57).
Treatment
The treatment strategies may be single or multimodal therapy with the goal of eradiation. This may be dictated by a variety of factors, such as AVM characteristics, operator skill, surgical or endovascular accessibility, venous drainage, and presence of high-risk features such as aneurysms.
Endovascular
Endovascular therapy has become increasingly popular with advances in technology, which include new microcatheter designs and development of solid and liquid embolic agents. Tortuous anatomy can now be overcome with superselective catheterization with microcatheters and flow-guided ultrathin microcatheters. Liquid embolic agents such as N-butyl cyanoacrylate (NBCA) and ethylene vinyl alcohol copolymer (Onyx) along with platinum embolic coils can be used to embolize AVMs (97-99).
In a meta-analysis of treatments of AVMs, endovascular embolization has a case fatality rate of 0.96 per 100 person years and hemorrhage rates of 1.7 per 100 person years. Complications leading to permanent neurologic deficits or death was seen in 6.6% of cases. With obliteration rates of 13% overall, but higher obliteration rates were seen in more recent cohorts (94).
Endovascular therapy can also be used in multimodal treatments to reduce the size or guide surgical or SRS planning (100). Some cases have shown curative embolization especially in small malformations. Long-term follow up is needed for definitive curative obliteration. Palliative embolization is also used in some cases for seizure control or stabilization of deficits in AVMs that are not amenable to SRS or surgical excision (100).
Benefits of endovascular therapy include it being minimally invasive, may provide immediate occlusion, and intraprocedural angiographic evaluation. Challenges that may be seen are incomplete embolization, recanalization, swelling or hemorrhage (101-105).
Surgical resection
Surgery requires a craniotomy and dural opening. For complete AVM resection, the nidus must be dissected circumferentially. First there must be careful devascularization with occlusion of the feeding arteries, then separation of the nidus from adjacent parenchyma, then division of draining veins (106).
SMG is used to estimate surgical risk of resection. Overall mortality rates are approximately 3.3% and morbidity rates are 8.6%, with increasing rates with increasing SMG. Typically for AVMs that are SMG 1 and 2, 92-100% have a favorable outcome. For SMG 3 AVMs, favorable short-term outcome is seen in 68.2% and long term in 8.6% of patients. For SMG 4 AVMs, favorable outcomes are seen in 73% and SMG 5 AVMs report good outcomes in 57.1% of patients (43).
Overall case fatality rates in a meta-analysis seen in 1.1 per 100 person years with hemorrhage rates of 0.18 per 100 person years. Complications leading to permanent neurologic deficits or death are seen in 7.4% and the highest obliteration rates for any therapy modality, approximately 96% (94,107-110).
Benefits include high rates of obliteration and immediate elimination of the risk of hemorrhage with obliteration, while challenges include intraoperative rupture, anatomic accessibility, edema from retraction, resection of normal tissue, and thrombosis of feeding vessels.
Stereotactic radiosurgery
A variety of radiotherapy methods have been used, but the most common is Gamma Knife, then linear accelerator and proton beam or helium ion. Obliteration occurs via endothelial damage and thickening of intimal layers followed by thrombosis and necrosis of AVM vessels, which take approximately 2-3 years with a median of 20 months for >95% obliteration (57,111-113). CT, MRI, and DSA are used to formulate radiosurgery treatment plans (114,115). Successful obliteration is based on a variety of factors, nidus volume and density, radiation dose, and location (112). Typically the dose will range from 18-25 Gy to the 50% isodose depending upon the adjacent area of the AVM.
Case fatality rates are lowest in this group with a rate of 0.5 per 100 person years. Hemorrhage rates for radiotherapy was similar to endovascular therapy, at 1.7 per 100 person years. Obliteration rates vary, overall it is approximately 38%, but outcomes are best in AVMs with a small volume in non-critical locations with high doses, which have obliteration rates >90% (94).
Studies of single-dose radiation therapy of large volume AVM have either high rates of complications or low obliteration rates. Obliterate rates ranged from 19% to 88% (116-124). Fractionated radiosurgery is typically used for treatment volumes greater than 10-15 mL.
In AVMs that do not completely obliterate with a single dose of SRS, repeat radiosurgery may be used for eventual obliteration, with rates up to 80%. Typically the residual AVM is reduced in size compared to the initially treated AVM (125). The results in the use of preradiosurgery embolization is mixed (126,127). There was an association with increased hemorrhage rates and higher risk of complications and possibly higher chance of obliteration (94,128).
A multicenter analysis reported that 8% of patients develop deficits after radiation (129). Early adverse effects include headache, nausea, and small risk of seizure in cortical AVMs treated in lobar regions. Delayed complications included neurologic deficits due to persistent edema, radiation necrosis, radiation-induced tumors, and cyst formation. These are increased with older age, large nidus, ruptured AVM, higher SMG AVM, and eloquent AVMs (129-134). In the Pittsburgh experience, 89% of patients who developed deficits with a minimum target dose less than 20 Gy, had complete resolution of their symptoms. Those with minimum target doses greater than 20 Gy, only 36% fully recovered (135).
The Pollock-Flickinger Score is a modified radiosurgery-based grading scale that can assist with predicting if patients will have any new neurologic deficits with treatment of AVMs with SRS. Obliteration without new neurologic deficits was seen in 90% for a score of less than 1 and 40% patients who scored greater than 2. For location, hemispheric, intraventricular, callosal and cerebellar AVMs are given a 0, while thalamic, basal ganglia, brainstem AVMs are given a 1.
(0.1×
Case example
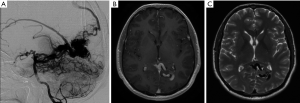
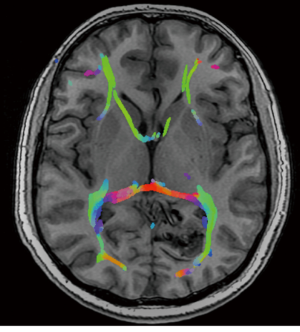
A previously healthy 35-year-old male, presented with visual seizures. A MRI and DSA was performed showing a 2.5 cm SMG 3 left occipital AVM, supplied by the posterior cerebral artery with a probably venous aneurysm and deep venous drainage (Figure 1). Functional MRI showed the nidus abutting the medial left optic radiation. The primary visual cortex was mapped 1 cm posterior to the nidus (Figure 2).
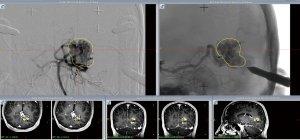
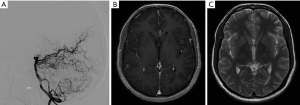
A planning MRI along with planning cerebral angiogram was performed. The patient underwent Gamma knife SRS with a prescription dose of 20 Gy to the 55% isodose line and treatment volume 4.6 mL (Figure 3). Postprocedure he had occasional headaches that resolved and he continued to have visual seizures, which were controlled with AEDs. The patient underwent follow up MRI at 2 years, which showed minimal flow voids with markedly reduced enhancement. A cerebral angiogram was performed showing no residual AVM (Figure 4).
Conclusions
AVMs are rare cerebral vascular lesions that can have devastating consequences. With the current knowledge of the natural history of AVMs, guidelines for management are unclear for asymptomatic unruptured AVMs. For AVMs that have ruptured, treatment is indicated due to the high risk of rerupture.
Treatment of large AVMs poses a challenge for surgical excision, embolization and radiosurgery. There are a variety of treatment modalities that can be used, with the goal for complete obliteration. Radiosurgery is a well-established and accepted standard in the treatment of AVMs. Small AVMS, typically with a volume less than 10 mL will require a single dose. Larger AVMs can be fractionated with good rates of obliteration. AVMs that are treated with partial obliteration can undergo repeat SRS with good obliteration rates. One thing to discuss with patients is that obliteration is gradual and will take approximately 2-3 years.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research for the series “SBRT/SRS in Radiation Research”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2014.07.07). The series “SBRT/SRS in Radiation Research” was commissioned by the editorial office without any funding or sponsorship. SV served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Doppman JL. The nidus concept of spinal cord arteriovenous malformations. A surgical recommendation based upon angiographic observations. Br J Radiol 1971;44:758-63. [PubMed]
- Virchow R. Die krankhaften Geschwulste. Berlin: A. Hirschwald, 1863:456-63.
- Steinheil SO. Ueber einen Fall von varix aneurysmaticus im Bereich der Gehirngefaesse. Würzburg: R. Fromme, 1895:1-56.
- Yasargil MG. AVM of the brain, history, embryology, pathological considerations, hemodynamics, diagnostic studies, microsurgical anatomy. Stuttgart: George Thieme Verlag, 1987.
- Lasjaunias P. A revised concept of the congenital nature of cerebral arteriovenous malformations. Interv Neuroradiol 1997;3:275-81. [PubMed]
- Mullan S, Mojtahedi S, Johnson DL, et al. Embryological basis of some aspects of cerebral vascular fistulas and malformations. J Neurosurg 1996;85:1-8. [PubMed]
- Deruty R, Pelissou-Guyotat I, Mottolese C, et al. The combined management of cerebral arteriovenous malformations. Experience with 100 cases and review of the literature. Acta Neurochir (Wien) 1993;123:101-12. [PubMed]
- Wohlwill FJ, Yakovlev PI. Histopathology of meningo-facial angiomatosis (Sturge-Weber’s disease); report of four cases. J Neuropathol Exp Neurol 1957;16:341-64. [PubMed]
- Stehbens W. Telangiectasias, hemangiomas, arteriovenous malformations, and allied disorders. In: Stehbens W. eds. Pathology of the Cerebral Blood Vessels. St. Louis: Mosby, 1972:471-558.
- Salcman M, Scholtz H, Numaguchi Y. Multiple intracerebral arteriovenous malformations: report of three cases and review of the literature. Surg Neurol 1992;38:121-8. [PubMed]
- Nussbaum ES, Heros RC, Madison MT, et al. The pathogenesis of arteriovenous malformations: insights provided by a case of multiple arteriovenous malformations developing in relation to a developmental venous anomaly. Neurosurgery 1998;43:347-51; discussion 351-2. [PubMed]
- McCormick WF. The pathology of vascular (“arteriovenous”) malformations. J Neurosurg 1966;24:807-16. [PubMed]
- Jellinger K. Vascular malformations of the central nervous system: a morphological overview. Neurosurg Rev 1986;9:177-216. [PubMed]
- Mawad ME, Hilal SK, Michelsen WJ, et al. Occlusive vascular disease associated with cerebral arteriovenous malformations. Radiology 1984;153:401-8. [PubMed]
- Mandybur TI, Nazek M. Cerebral arteriovenous malformations. A detailed morphological and immunohistochemical study using actin. Arch Pathol Lab Med 1990;114:970-3. [PubMed]
- Cunha e Sa MJ, Stein BM, Solomon RA, et al. The treatment of associated intracranial aneurysms and arteriovenous malformations. J Neurosurg 1992;77:853-9. [PubMed]
- Lasjaunias P, Piske R, Terbrugge K, et al. Cerebral arteriovenous malformations (C. AVM) and associated arterial aneurysms (AA). Analysis of 101 C. AVM cases, with 37 AA in 23 patients. Acta Neurochir (Wien) 1988;91:29-36. [PubMed]
- Meisel HJ, Mansmann U, Alvarez H, et al. Cerebral arteriovenous malformations and associated aneurysms: analysis of 305 cases from a series of 662 patients. Neurosurgery 2000;46:793-800; discussion 800-2. [PubMed]
- Ogilvy CS, Stieg PE, Awad I, et al. Recommendations for the management of intracranial arteriovenous malformations: a statement for healthcare professionals from a special writing group of the Stroke Council, American Stroke Association. Circulation 2001;103:2644-57. [PubMed]
- Chin LS, Raffel C, Gonzalez-Gomez I, et al. Diffuse arteriovenous malformations: a clinical, radiological, and pathological description. Neurosurgery 1992;31:863-8; discussion 868-9. [PubMed]
- Viñuela F, Nombela L, Roach MR, et al. Stenotic and occlusive disease of the venous drainage system of deep brain AVM’s. J Neurosurg 1985;63:180-4. [PubMed]
- Challa VR, Moody DM, Brown WR, et al. Vascular malformations of the central nervous system. J Neuropathol Exp Neurol 1995;54:609-21. [PubMed]
- Vander Eecken HM, Adams RD. The anatomy and functional significance of the meningeal arterial anastomoses of the human brain. J Neuropathol Exp Neurol 1953;12:132-57. [PubMed]
- Stapf C, Mohr JP, Sciacca RR, et al. Incident hemorrhage risk of brain arteriovenous malformations located in the arterial borderzones. Stroke 2000;31:2365-8. [PubMed]
- Jeffree RL, Stoodley MA. Postnatal development of arteriovenous malformations. Pediatr Neurosurg 2009;45:296-304. [PubMed]
- Moftakhar P, Hauptman JS, Malkasian D, et al. Cerebral arteriovenous malformations. Part 1: cellular and molecular biology. Neurosurg Focus 2009;26:E10 [PubMed]
- Deshpande DH, Vidyasagar C. Histology of the persistent embryonic veins in arteriovenous malformations of brain. Acta Neurochir (Wien) 1980;53:227-36. [PubMed]
- Mullan S. Reflections upon the nature and management of intracranial and intraspinal vascular malformations and fistulae. J Neurosurg 1994;80:606-16. [PubMed]
- Mullan S, Mojtahedi S, Johnson DL, et al. Cerebral venous malformation-arteriovenous malformation transition forms. J Neurosurg 1996;85:9-13. [PubMed]
- Streeter G. The development of the venous sinus of the dura mater in the human embryo. Am J Anat 1915;18:145-78.
- Streeter GL. The developmental alterations in the vascular system of the brain of the human embryo. Contrib Embryol 1918;8:5-38.
- Bederson JB, Wiestler OD, Brüstle O, et al. Intracranial venous hypertension and the effects of venous outflow obstruction in a rat model of arteriovenous fistula. Neurosurgery 1991;29:341-50. [PubMed]
- Herman JM, Spetzler RF, Bederson JB, et al. Genesis of a dural arteriovenous malformation in a rat model. J Neurosurg 1995;83:539-45. [PubMed]
- Lawton MT, Jacobowitz R, Spetzler RF. Redefined role of angiogenesis in the pathogenesis of dural arteriovenous malformations. J Neurosurg 1997;87:267-74. [PubMed]
- Lim M, Cheshier S, Steinberg GK. New vessel formation in the central nervous system during tumor growth, vascular malformations, and Moyamoya. Curr Neurovasc Res 2006;3:237-45. [PubMed]
- Shenkar R, Elliott JP, Diener K, et al. Differential gene expression in human cerebrovascular malformations. Neurosurgery 2003;52:465-77; discussion 477-8. [PubMed]
- Koizumi T, Shiraishi T, Hagihara N, et al. Expression of vascular endothelial growth factors and their receptors in and around intracranial arteriovenous malformations. Neurosurgery 2002;50:117-24; discussion 124-6. [PubMed]
- Minakawa T, Tanaka R, Koike T, et al. Angiographic follow-up study of cerebral arteriovenous malformations with reference to their enlargement and regression. Neurosurgery 1989;24:68-74. [PubMed]
- Kondziolka D, Humphreys RP, Hoffman HJ, et al. Arteriovenous malformations of the brain in children: a forty year experience. Can J Neurol Sci 1992;19:40-5. [PubMed]
- Hashimoto T, Lam T, Boudreau NJ, et al. Abnormal balance in the angiopoietin-tie2 system in human brain arteriovenous malformations. Circ Res 2001;89:111-3. [PubMed]
- Berg JN, Guttmacher AE, Marchuk DA, et al. Clinical heterogeneity in hereditary haemorrhagic telangiectasia: are pulmonary arteriovenous malformations more common in families linked to endoglin? J Med Genet 1996;33:256-7. [PubMed]
- Cymerman U, Vera S, Pece-Barbara N, et al. Identification of hereditary hemorrhagic telangiectasia type 1 in newborns by protein expression and mutation analysis of endoglin. Pediatr Res 2000;47:24-35. [PubMed]
- Speizler RF, Martin NA. A proposed grading system for arteriovenous malformations. 1986. J Neurosurg 2008;108:186-93. [PubMed]
- Berman MF, Sciacca RR, Pile-Spellman J, et al. The epidemiology of brain arteriovenous malformations. Neurosurgery 2000;47:389-96; discussion 397. [PubMed]
- Al-Shahi R, Fang JS, Lewis SC, et al. Prevalence of adults with brain arteriovenous malformations: a community based study in Scotland using capture-recapture analysis. J Neurol Neurosurg Psychiatry 2002;73:547-51. [PubMed]
- Al-Shahi R, Bhattacharya JJ, Currie DG, et al. Prospective, population-based detection of intracranial vascular malformations in adults: the Scottish Intracranial Vascular Malformation Study (SIVMS). Stroke 2003;34:1163-9. [PubMed]
- Stapf C, Mast H, Sciacca RR, et al. The New York Islands AVM Study: design, study progress, and initial results. Stroke 2003;34:e29-33. [PubMed]
- Morris Z, Whiteley WN, Longstreth WT Jr, et al. Incidental findings on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ 2009;339:b3016. [PubMed]
- Jessurun GA, Kamphuis DJ, van der Zande FH, et al. Cerebral arteriovenous malformations in The Netherlands Antilles. High prevalence of hereditary hemorrhagic telangiectasia-related single and multiple cerebral arteriovenous malformations. Clin Neurol Neurosurg 1993;95:193-8. [PubMed]
- Brown RD Jr, Wiebers DO, Torner JC, et al. Frequency of intracranial hemorrhage as a presenting symptom and subtype analysis: a population-based study of intracranial vascular malformations in Olmsted Country, Minnesota. J Neurosurg 1996;85:29-32. [PubMed]
- Perret G, Nishioka H. Report on the cooperative study of intracranial aneurysms and subarachnoid hemorrhage. Section VI. Arteriovenous malformations. An analysis of 545 cases of cranio-cerebral arteriovenous malformations and fistulae reported to the cooperative study. J Neurosurg 1966;25:467-90. [PubMed]
- Stapf C, Labovitz DL, Sciacca RR, et al. Incidence of adult brain arteriovenous malformation hemorrhage in a prospective population-based stroke survey. Cerebrovasc Dis 2002;13:43-6. [PubMed]
- da Costa L, Wallace MC, Ter Brugge KG, et al. The natural history and predictive features of hemorrhage from brain arteriovenous malformations. Stroke 2009;40:100-5. [PubMed]
- Pollock BE, Flickinger JC, Lunsford LD, et al. Factors that predict the bleeding risk of cerebral arteriovenous malformations. Stroke 1996;27:1-6. [PubMed]
- Stapf C, Mast H, Sciacca RR, et al. Predictors of hemorrhage in patients with untreated brain arteriovenous malformation. Neurology 2006;66:1350-5. [PubMed]
- Fults D, Kelly DL Jr. Natural history of arteriovenous malformations of the brain: a clinical study. Neurosurgery 1984;15:658-62. [PubMed]
- Starke RM, Komotar RJ, Connolly ES. A randomized trial of unruptured brain arteriovenous malformations. Neurosurgery 2013;73:N13-5.
- Hofmeister C, Stapf C, Hartmann A, et al. Demographic, morphological, and clinical characteristics of 1289 patients with brain arteriovenous malformation. Stroke 2000;31:1307-10. [PubMed]
- Ondra SL, Troupp H, George ED, et al. The natural history of symptomatic arteriovenous malformations of the brain: a 24-year follow-up assessment. J Neurosurg 1990;73:387-91. [PubMed]
- Mckissock W, Paterson JH. A clinical survey of intracranial angiomas with special reference to their mode of progression and surgical treatment: a report of 110 cases. Brain 1956;79:233-66. [PubMed]
- Henderson WR, Gomez RD. Natural history of cerebral angiomas. Br Med J 1967;4:571-4. [PubMed]
- Houser OW, Baker HL Jr, Svien HJ, et al. Arteriovenous malformations of the parenchyma of the brain. Angiographic aspects. Radiology 1973;109:83-90. [PubMed]
- Waltimo O. The relationship of size, density and localization of intracranial arteriovenous malformations to the type of initial symptom. J Neurol Sci 1973;19:13-9. [PubMed]
- Guidetti B, Delitala A. Intracranial arteriovenous malformations. Conservative and surgical treatment. J Neurosurg 1980;53:149-52. [PubMed]
- Parkinson D, Bachers G. Arteriovenous malformations. Summary of 100 consecutive supratentorial cases. J Neurosurg 1980;53:285-99. [PubMed]
- Graf CJ, Perret GE, Torner JC. Bleeding from cerebral arteriovenous malformations as part of their natural history. J Neurosurg 1983;58:331-7. [PubMed]
- Crawford PM, West CR, Chadwick DW, et al. Arteriovenous malformations of the brain: natural history in unoperated patients. J Neurol Neurosurg Psychiatry 1986;49:1-10. [PubMed]
- Itoyama Y, Uemura S, Ushio Y, et al. Natural course of unoperated intracranial arteriovenous malformations: study of 50 cases. J Neurosurg 1989;71:805-9. [PubMed]
- Spetzler RF, Hargraves RW, McCormick PW, et al. Relationship of perfusion pressure and size to risk of hemorrhage from arteriovenous malformations. J Neurosurg 1992;76:918-23. [PubMed]
- Hernesniemi JA, Dashti R, Juvela S, et al. Natural history of brain arteriovenous malformations: a long-term follow-up study of risk of hemorrhage in 238 patients. Neurosurgery 2008;63:823-9; discussion 829-31. [PubMed]
- Mast H, Young WL, Koennecke HC, et al. Risk of spontaneous haemorrhage after diagnosis of cerebral arteriovenous malformation. Lancet 1997;350:1065-8. [PubMed]
- Miyasaka Y, Yada K, Ohwada T, et al. An analysis of the venous drainage system as a factor in hemorrhage from arteriovenous malformations. J Neurosurg 1992;76:239-43. [PubMed]
- Mansmann U, Meisel J, Brock M, et al. Factors associated with intracranial hemorrhage in cases of cerebral arteriovenous malformation. Neurosurgery 2000;46:272-9; discussion 279-81. [PubMed]
- Wilkins RH. Natural history of intracranial vascular malformations: a review. Neurosurgery 1985;16:421-30. [PubMed]
- Choi JH, Mast H, Sciacca RR, et al. Clinical outcome after first and recurrent hemorrhage in patients with untreated brain arteriovenous malformation. Stroke 2006;37:1243-7. [PubMed]
- Hartmann A, Mast H, Mohr JP, et al. Morbidity of intracranial hemorrhage in patients with cerebral arteriovenous malformation. Stroke 1998;29:931-4. [PubMed]
- Norris JS, Valiante TA, Wallace MC, et al. A simple relationship between radiological arteriovenous malformation hemodynamics and clinical presentation: a prospective, blinded analysis of 31 cases. J Neurosurg 1999;90:673-9. [PubMed]
- Osipov A, Koennecke HC, Hartmann A, et al. Seizures in cerebral arteriovenous malformations: type, clinical course, and medical management. Interv Neuroradiol 1997;3:37-41. [PubMed]
- Lees F. The migrainous symptoms of cerebral angiomata. J Neurol Neurosurg Psychiatry 1962;25:45-50. [PubMed]
- Homan RW, Devous MD Sr, Stokely EM, et al. Quantification of intracerebral steal in patients with arteriovenous malformation. Arch Neurol 1986;43:779-85. [PubMed]
- Okabe T, Meyer JS, Okayasu H, et al. Xenon-enhanced CT CBF measurements in cerebral AVM’s before and after excision. Contribution to pathogenesis and treatment. J Neurosurg 1983;59:21-31. [PubMed]
- Mahalick DM, Ruff RM. U HS. Neuropsychological sequelae of arteriovenous malformations. Neurosurgery 1991;29:351-7. [PubMed]
- Mast H, Mohr JP, Thompson JL, et al. Transcranial Doppler ultrasonography in cerebral arteriovenous malformations. Diagnostic sensitivity and association of flow velocity with spontaneous hemorrhage and focal neurological deficit. Stroke 1995;26:1024-7. [PubMed]
- Mast H, Mohr JP, Osipov A, et al. ‘Steal’ is an unestablished mechanism for the clinical presentation of cerebral arteriovenous malformations. Stroke 1995;26:1215-20. [PubMed]
- Young WL, Pile-Spellman J, Prohovnik I, et al. Evidence for adaptive autoregulatory displacement in hypotensive cortical territories adjacent to arteriovenous malformations. Columbia University AVM Study Project. Neurosurgery 1994;34:601-10; discussion 610-11. [PubMed]
- Brown RD Jr, Flemming KD, Meyer FB, et al. Natural history, evaluation, and management of intracranial vascular malformations. Mayo Clin Proc 2005;80:269-81. [PubMed]
- Mossa-Basha M, Chen J, Gandhi D. Imaging of cerebral arteriovenous malformations and dural arteriovenous fistulas. Neurosurg Clin N Am 2012;23:27-42. [PubMed]
- Friedlander RM. Clinical practice. Arteriovenous malformations of the brain. N Engl J Med 2007;356:2704-12. [PubMed]
- Eddleman CS, Jeong HJ, Hurley MC, et al. 4D radial acquisition contrast-enhanced MR angiography and intracranial arteriovenous malformations: quickly approaching digital subtraction angiography. Stroke 2009;40:2749-53. [PubMed]
- Latchaw RE, Hu X, Ugurbil K, et al. Functional magnetic resonance imaging as a management tool for cerebral arteriovenous malformations. Neurosurgery 1995;37:619-25; discussion 625-6. [PubMed]
- Petrella JR, Shah LM, Harris KM, et al. Preoperative functional MR imaging localization of language and motor areas: effect on therapeutic decision making in patients with potentially resectable brain tumors. Radiology 2006;240:793-802. [PubMed]
- Ulmer JL, Krouwer HG, Mueller WM, et al. Pseudo-reorganization of language cortical function at fMR imaging: a consequence of tumor-induced neurovascular uncoupling. AJNR Am J Neuroradiol 2003;24:213-7. [PubMed]
- Turjman F, Massoud TF, Viñuela F, et al. Aneurysms related to cerebral arteriovenous malformations: superselective angiographic assessment in 58 patients. AJNR Am J Neuroradiol 1994;15:1601-5. [PubMed]
- van Beijnum J, van der Worp HB, Buis DR, et al. Treatment of brain arteriovenous malformations: a systematic review and meta-analysis. JAMA 2011;306:2011-9. [PubMed]
- Mohr JP, Moskowitz AJ, Stapf C, et al. The ARUBA trial: current status, future hopes. Stroke 2010;41:e537-40. [PubMed]
- Mohr JP, Parides MK, Stapf C, et al. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet 2014;383:614-21. [PubMed]
- Berenstein AB, Krall R, Choi IS. Embolization with n-butyl cyanoacrylate in the management of CNS vascular lesions. Am J Neuroradiol 1989;10:883.
- Wallace RC, Flom RA, Khayata MH, et al. The safety and effectiveness of brain arteriovenous malformation embolization using acrylic and particles: the experiences of a single institution. Neurosurgery 1995;37:606-15; discussion 615-8. [PubMed]
- Yu SC, Chan MS, Lam JM, et al. Complete obliteration of intracranial arteriovenous malformation with endovascular cyanoacrylate embolization: initial success and rate of permanent cure. AJNR Am J Neuroradiol 2004;25:1139-43. [PubMed]
- Pelz DM, Fox AJ, Viñuela F, et al. Preoperative embolization of brain AVMs with isobutyl-2 cyanoacrylate. AJNR Am J Neuroradiol 1988;9:757-64. [PubMed]
- Ledezma CJ, Hoh BL, Carter BS, et al. Complications of cerebral arteriovenous malformation embolization: multivariate analysis of predictive factors. Neurosurgery 2006;58:602-11; discussion 602-11. [PubMed]
- Rao VR, Mandalam KR, Gupta AK, et al. Dissolution of isobutyl 2-cyanoacrylate on long-term follow-up. AJNR Am J Neuroradiol 1989;10:135-41. [PubMed]
- Fournier D, Terbrugge K, Rodesch G, et al. Revascularization of brain arteriovenous malformations after embolization with bucrylate. Neuroradiology 1990;32:497-501. [PubMed]
- Fournier D, TerBrugge KG, Willinsky R, et al. Endovascular treatment of intracerebral arteriovenous malformations: experience in 49 cases. J Neurosurg 1991;75:228-33. [PubMed]
- Wikholm G. Occlusion of cerebral arteriovenous malformations with N-butyl cyano-acrylate is permanent. AJNR Am J Neuroradiol 1995;16:479-82. [PubMed]
- Pradilla G, Coon AL, Huang J, et al. Surgical treatment of cranial arteriovenous malformations and dural arteriovenous fistulas. Neurosurg Clin N Am 2012;23:105-22. [PubMed]
- Heros RC, Korosue K, Diebold PM. Surgical excision of cerebral arteriovenous malformations: late results. Neurosurgery 1990;26:570-7; discussion 577-8. [PubMed]
- Sisti MB, Kader A, Stein BM. Microsurgery for 67 intracranial arteriovenous malformations less than 3 cm in diameter. J Neurosurg 1993;79:653-60. [PubMed]
- Hamilton MG, Spetzler RF. The prospective application of a grading system for arteriovenous malformations. Neurosurgery 1994;34:2-6; discussion 6-7. [PubMed]
- Schaller C, Schramm J, Haun D. Significance of factors contributing to surgical complications and to late outcome after elective surgery of cerebral arteriovenous malformations. J Neurol Neurosurg Psychiatry 1998;65:547-54. [PubMed]
- Schneider BF, Eberhard DA, Steiner LE. Histopathology of arteriovenous malformations after gamma knife radiosurgery. J Neurosurg 1997;87:352-7. [PubMed]
- Starke RM, Komotar RJ, Hwang BY, et al. A comprehensive review of radiosurgery for cerebral arteriovenous malformations: outcomes, predictive factors, and grading scales. Stereotact Funct Neurosurg 2008;86:191-9. [PubMed]
- International RadioSurgery Association (IRSA). Stereotactic radiosurgery for patients with intracranial arteriovenous malformations (AVM). Harrisburg (PA): International RadioSurgery Association (IRSA); 2009 Mar. 22 p. (Radiosurgery practice guideline report; no. 2-03).
- Bednarz G, Downes B, Werner-Wasik M, et al. Combining stereotactic angiography and 3D time-of-flight magnetic resonance angiography in treatment planning for arteriovenous malformation radiosurgery. Int J Radiat Oncol Biol Phys 2000;46:1149-54. [PubMed]
- Berger MO, Anxionnat R, Kerrien E, et al. A methodology for validating a 3D imaging modality for brain AVM delineation: application to 3DRA. Comput Med Imaging Graph 2008;32:544-53. [PubMed]
- Steinberg GK, Fabrikant JI, Marks MP, et al. Stereotactic heavy-charged-particle Bragg-peak radiation for intracranial arteriovenous malformations. N Engl J Med 1990;323:96-101. [PubMed]
- Lunsford LD, Kondziolka D, Flickinger JC, et al. Stereotactic radiosurgery for arteriovenous malformations of the brain. J Neurosurg 1991;75:512-24. [PubMed]
- Steiner L, Lindquist C, Adler JR, et al. Clinical outcome of radiosurgery for cerebral arteriovenous malformations. J Neurosurg 1992;77:1-8. [PubMed]
- Colombo F, Pozza F, Chierego G, et al. Linear accelerator radiosurgery of cerebral arteriovenous malformations: an update. Neurosurgery 1994;34:14-20; discussion 20-1. [PubMed]
- Friedman WA, Bova FJ, Mendenhall WM. Linear accelerator radiosurgery for arteriovenous malformations: the relationship of size to outcome. J Neurosurg 1995;82:180-9. [PubMed]
- Yamamoto Y, Coffey RJ, Nichols DA, et al. Interim report on the radiosurgical treatment of cerebral arteriovenous malformations. The influence of size, dose, time, and technical factors on obliteration rate. J Neurosurg 1995;83:832-7. [PubMed]
- Pollock BE, Flickinger JC, Lunsford LD, et al. Hemorrhage risk after stereotactic radiosurgery of cerebral arteriovenous malformations. Neurosurgery 1996;38:652-9; discussion 659-61. [PubMed]
- Schlienger M, Atlan D, Lefkopoulos D, et al. Linac radiosurgery for cerebral arteriovenous malformations: results in 169 patients. Int J Radiat Oncol Biol Phys 2000;46:1135-42. [PubMed]
- Flickinger JC, Kondziolka D, Maitz AH, et al. An analysis of the dose-response for arteriovenous malformation radiosurgery and other factors affecting obliteration. Radiother Oncol 2002;63:347-54. [PubMed]
- Kano H, Kondziolka D, Flickinger JC, et al. Stereotactic radiosurgery for arteriovenous malformations, Part 3: outcome predictors and risks after repeat radiosurgery. J Neurosurg 2012;116:21-32. [PubMed]
- Gobin YP, Laurent A, Merienne L, et al. Treatment of brain arteriovenous malformations by embolization and radiosurgery. J Neurosurg 1996;85:19-28. [PubMed]
- Henkes H, Nahser HC, Berg-Dammer E, et al. Endovascular therapy of brain AVMs prior to radiosurgery. Neurol Res 1998;20:479-92. [PubMed]
- Zabel-du Bois A, Milker-Zabel S, Huber P, et al. Risk of hemorrhage and obliteration rates of LINAC-based radiosurgery for cerebral arteriovenous malformations treated after prior partial embolization. Int J Radiat Oncol Biol Phys 2007;68:999-1003. [PubMed]
- Flickinger JC, Kondziolka D, Lunsford LD, et al. A multi-institutional analysis of complication outcomes after arteriovenous malformation radiosurgery. Int J Radiat Oncol Biol Phys 1999;44:67-74. [PubMed]
- Kihlström L, Guo WY, Karlsson B, et al. Magnetic resonance imaging of obliterated arteriovenous malformations up to 23 years after radiosurgery. J Neurosurg 1997;86:589-93. [PubMed]
- Pollock BE, Flickinger JC, Lunsford LD, et al. Factors associated with successful arteriovenous malformation radiosurgery. Neurosurgery 1998;42:1239-44; discussion 1244-7. [PubMed]
- Hara M, Nakamura M, Shiokawa Y, et al. Delayed cyst formation after radiosurgery for cerebral arteriovenous malformation: two case reports. Minim Invasive Neurosurg 1998;41:40-5. [PubMed]
- Kim MS, Lee SI, Sim JH. A case of very large cyst formation with Gamma Knife radiosurgery for an arteriovenous malformation. Stereotact Funct Neurosurg 1999;72:168-74. [PubMed]
- Flickinger JC, Kondziolka D, Lunsford LD, et al. Development of a model to predict permanent symptomatic postradiosurgery injury for arteriovenous malformation patients. Arteriovenous Malformation Radiosurgery Study Group. Int J Radiat Oncol Biol Phys 2000;46:1143-8. [PubMed]
- Flickinger JC, Kondziolka D, Pollock BE, et al. Complications from arteriovenous malformation radiosurgery: multivariate analysis and risk modeling. Int J Radiat Oncol Biol Phys 1997;38:485-90. [PubMed]


