A case report of small bowel adenocarcinoma with liver metastases: genetic profiling and clinical management
Introduction
Small bowel adenocarcinoma (SBA) is usually a rare and fatal gastrointestinal malignancy, which represents a third of all small bowel cancers (1). Although rare, its incidence is rising with an estimated 10,190 new cases reported in the United States in 2017 (1-3). Due to its non-specific and heterogeneous presentation, most SBA patients are diagnosed with late-stage disease (4). Surgical resection is the optimal therapy for localized and regional disease. In patients with clinically advanced disease, several retrospective studies have demonstrated that patients receiving systemic chemotherapy attain clinical benefits with a median overall survival of 13 months when compared with only 4 months in patients with best-supportive care (4). To date, no standard treatment guideline has been established for advanced SBA. Therefore, clinical management of metastatic SBA is very challenging. However, based on multiple prospective studies, oxaliplatin-based chemotherapy regimens are most frequently used to treat advanced SBA (5,6). Recently, Horimatsu et al. reported a median progression-free survival (PFS) of 5.4 and a median overall survival (OS) of 17.3 months in Japanese patients with advanced SBA treated with mFOLFOX6 (7). Targeted agents such as bevacizumab and cetuximab have improved clinical outcomes for CRC patients, and may have clinical activity in SBA, which has high levels of vascular endothelial growth factor receptor A (VEGFR-A, 96%) and epidermal growth factor receptor (EGFR, 71%) (8). The first prospective study that evaluated the efficacy of CAPOX with bevacizumab in patients with SBA and AAC (ampullary adenocarcinoma) demonstrated an effective and well-tolerated potential therapy with a median PFS of 8.7 months and a median OS of 12.9 months (9). In addition, Aydin et al. evaluated the efficacy of bevacizumab along with the chemotherapy regimens FOLFOX and FOLFIRI and demonstrated greater clinical efficacy than chemotherapy alone (10). However, treatment outcome in advanced SBA patients is still unsatisfactory. Collaborative clinical trials are needed to improve treatment outcome in this orphan tumor. In this study, we report a case of an advanced small bowel adenocarcinoma patient with a long PFS of more than 3 years, whose treatment plan was tailored by analyzing her genetic background and drug sensitivity.
Case presentation
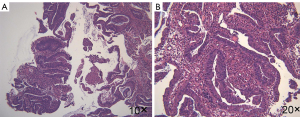
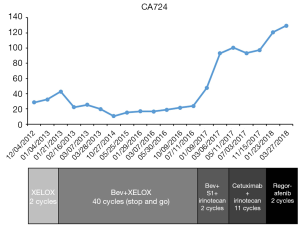
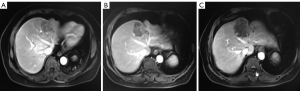
The patient agreed and signed informed consent for publication of her data. This study was carried out in accordance with the Institutional Ethics Committee of Shanghai Tenth People’s Hospital. A 70-year-old woman was transferred to Shanghai Tenth People’s Hospital complaining of right middle abdominal pain. A mass in the distal duodenum was identified after performing a gastroscopy. Pathological examination revealed poorly differentiated duodenal adenocarcinoma (Figure 1). Whole-body positron emission tomography-computed tomography (PET-CT) examination suggested multiple liver metastases (Figure S1), and the patient underwent transcatheter arterial chemoembolization (TACE) for the liver metastases. Therefore, the patient was diagnosed at stage IV. Genetic testing was performed in this patient to predict prognosis and guide clinical management. Next-generation sequencing (NGS) detected 35 gene mutations, small insertions/deletions or gene fusions related to targeted therapy or prognosis (Table S1). single-nucleotide polymorphisms (SNPs) of 13 genes associated with chemotherapy agents were also tested (Table 1). A gene test indicated that the patient was sensitive to oxaliplatin and fluorouracil. Based on the gene detection results and our experience, we treated her with palliative chemotherapy with two courses of XELOX (oxaliplatin 130 mg/m2 d1 + capecitabine 1 g/m2 d1–14 q3w). Tumor assessment by MRI showed stable disease. However, tumor markers (CA724: 42.86) were elevated. Therefore, using this information in conjunction with our experience in CRC, the patient was treated with XELOX plus bevacizumab (7.5 mg/kg) combination therapy. After 6 cycles of treatment, the patient disease status was stable with good tolerance of this regimen. Therefore, we continued this regimen as maintenance therapy with a longer treatment interval (every 4–6 weeks). Considering the neurotoxicity of oxaliplatin, stop and go policy was applied with oxaliplatin removed from cycle 16 to cycle 30 and added again in the next 10 cycles. After 20 courses of regimens (January 24, 2013 to June 18, 2014), the CA724 tumor marker decreased and was stable with continued treatment until disease progression (Figure 2). The treatment was well-tolerated with limited adverse effects. Totally, the patient underwent 40 cycles XELOX plus bevacizumab using stop and go policy for more than 3 years with stable disease from January 24, 2013 to November 24, 2016. The patient experienced Grade 3 proteinuria and Grade 2 neurotoxicity. An MRI scan on January 9, 2017 revealed disease progression of the liver lesions (Figure 3). Therefore, the patient received two courses of bevacizumab plus tegafur, gimeracil, and oteracil potassium capsules (50 mg BID d1–14) with irinotecan (180 mg/m2). She then underwent one course of high-intensity focused ultrasound (HIFU) therapy for the liver lesions. As shown in Table S1, no KRAS or BRAF mutations were detected, and therefore, the patient received targeted therapy with cetuximab (500 mg/m2 q3w) combined with irinotecan with a PFS of 10 months. However, the patient progressed again in January 2018. She was then treated with regorafenib, which is a third-line treatment option for patients with metastatic colorectal cancer. The patient died three months later because of a heart attack.
Table 1
| Gene | Detection site | Result | Clinical significance | ||
|---|---|---|---|---|---|
| Chemotherapy agent | Response rate | Side effects | |||
| ERCC1 | Codon 118 polymorphisms | Wild | Platinum drugs | High | Low |
| ERCC2 | Codon 751 polymorphisms | Wild | |||
| GSTP1 | Exon 5 A313G polymorphisms | Homozygous mutation | |||
| XRCC1 | Exon6 Arg194Trp polymorphisms | Wild | |||
| Exon10 Codon 399 polymorphisms | Wild | ||||
| DPYD | DPYD*2A polymorphisms | Wild | 5-FU/ capecitabine | High | Media |
| DPYD*5A polymorphisms | Heterozygous mutation | ||||
| DPYD*9A polymorphisms | Wild | ||||
| MTHFR | C677T polymorphisms | Homozygous mutation | |||
| GSTT1 | Genetic defect | Wild | |||
| UGT1A1 | UGT1A1*6 | Wild | Irinotecan | – | Low |
| UGT1A1*28 | Wild | ||||
ERCC1, ERCC excision repair 1, endonuclease non-catalytic subunit; ERCC2, ERCC excision repair 2, TFIIH core complex helicase subunit; GSTP1, glutathione S-transferase pi 1; XRCC1, X-ray repair cross complementing 1; DPYD, dihydropyrimidine dehydrogenase; MTHFR, methylenetetrahydrofolate reductase; GSTT1, glutathione S-transferase theta 1; UGT1A1, UDP glucuronosyltransferase family 1 member A1.
Discussion
The treatment deficiency and rarity of SBA have hampered the understanding of the molecular factors that drive this malignancy and have resulted in poor patient outcomes. In the past, the treatment regimen for advanced colorectal or gastric cancer has been applied to advanced SBA. However, there are significant molecular differences in these tumor types, which call for distinct treatment strategies. Currently, there is no standard of care for the treatment of advanced SBA. Current therapies have been guided by phase II and retrospective studies. Therefore, randomized clinical trials are sorely needed to guide treatment and improve outcome. To date, there have been no randomized clinical trials comparing the effectiveness of different chemotherapy regimens in SBA. However, phase II prospective studies have recommended the use of either CAPOX or FOLFOX as the standard first-line therapy for SBA (7).
Due to the lack of references regarding chemotherapy sensitivity in SBA, we chose a chemotherapy regimen based on colorectal cancer data. Several genes, including nucleotide excision DNA repair cross-complementation groups 1 and 2 (ERCC1 and ERCC2), glutathione S-transferase (GSTP1), and X-ray cross-complementing group 1 (XRCC1), carry well-known polymorphisms associated with oxaliplatin-based chemotherapy toxicity (11). This patient had GSTP1 313A>G in exon 5, which was reported to be associated with reduced enzyme activity, anticancer drug resistance, and oxaliplatin toxicity. Multiple studies have demonstrated that ERCC1 codon 118 polymorphism may affect the mRNA and its protein expression levels, resulting in differences in platinum sensitivity and clinical outcome (12). Improved clinical outcome and higher platinum sensitivity have been seen with ERCC1-118 C/C homozygote genotype. For ERCC2, patients with A/A (Lys/Lys) genotype at codon 751 were associated with better response, longer PFS and OS in comparison to those with C/C and the heterozygous A/C (13). Patients carrying Gln mutant allele at codon 399 of XRCC1 (heterozygous plus homozygous) were more likely to experience unsuccessful chemotherapy with 5-FU/oxaliplatin due to resistance (14). Based on this information, we surmised that this patient was sensitive to oxaliplatin. Genes with SNPs associated with fluorouracil-induced toxicities include DPYD and MTHFR. The most relevant polymorphism in DPYD is DPYD*2A. DPYD*5A and DPYD*9A mutants also frequently occur in the Chinese population (15). All the mutants can lead to higher toxicity of fluorouracil because of reduced enzyme activity. This patient had a heterozygous DPYD*5A mutant, but we did not observe obvious adverse effects of fluorouracil. In vitro, MTHFR 677T mutation in CRC increased 5-FU activity and MTHFR 677 CT+TT genotypes were associated with improved OS compared with those the MTHFR 677 CC genotype (16). In this patient, 5-FU-based chemotherapy was beneficial and well tolerated.
Nevertheless, VEGF-A (96%) and EGFR (71%) expressions are high in SBA (8). A limited number of case reports have documented encouraging results with the addition of targeted therapy to chemotherapy for the treatment of SBA. A dynamic case report recently demonstrated imperative results in patients with advanced SBA treated with bevacizumab in combination with gemcitabine and oxaliplatin, and finally maintained on bevacizumab plus capecitabine (17). Another case report showed prolonged disease-free survival in one patient with recurrent SBA after treatment with bevacizumab in combination with FOLFOX-6 (18). In addition, Takayoshi et al. reported chemotherapy in combination with bevacizumab has provided a favorable result when compared to chemotherapy alone (19). Therefore, a combination of bevacizumab and chemotherapy may be an option for advanced small bowel adenocarcinoma. Prospective studies are now in progress to explore multiple targeted therapies in advanced small bowel adenocarcinoma. There are also a few case reports, which have indicated that anti-epidermal growth factor receptor therapy (e.g., cetuximab and panitumumab) may be effective in SBA (20,21). A PFS of 19 months was achieved with panitumumab as a maintenance therapy (22). In our case, the patient chose bevacizumab as initial target treatment due to financial constraints. And she also benefited from cetuximab when she progressed with bevacizumab.
In comparison to large bowel cancer, the genetic changes of which have been well investigated, little research has been done on the carcinogenesis of small bowel cancer. The genetic alterations principal to colorectal cancer carcinogenesis have been investigated in SBA. A cohort study of 194 patients with SBA indicated that aberrant activation of the Wnt/APC and β-catenin pathway was correlated with poor prognosis. However, mutations in adenomatous polyposis coli (APC) occur in only 10–15% of cases of sporadic SBA (23). Loss of the distal 18q including the SMAD4 and DCC genes occurs in approximately 73% of sporadic colorectal cancers and 47% of SBA. In contrast to DCC, mutations in the tumor suppressor gene SMAD4, which is involved in the TGF-β signaling pathway, are more common in small bowel adenocarcinomas (30%) (24). The prevalence of KRAS mutations in sporadic SBA is 40–60%, comparable to that in colorectal cancers. However, BRAF V600E mutations are rare in SBA. In addition, tumor protein 53 (TP53) gene mutations and p53 isoform overexpression are seen in 30–60% of SBA. TP53 mutations are associated with poor survival. Laforest et al. showed that 12% of SBA patients had a tumor alteration in HER-2 (25). Genetic testing of this patient was performed to aide prediction of prognosis and clinical management of the disease. No mutations were detected in APC, KRAS, NRAS, BRAF, TP53, and HER-2. The genetic makeup of this patient may have influenced her treatment response and contributed to her better prognosis.
Conclusions
Our case indicates the potential effective therapy of XELOX plus bevacizumab for SBA. This case report is the first to explore the efficacy of bevacizumab in combination with XELOX as a maintenance therapy. The sustained clinical benefit was observed in a patient with advanced SBA, who showed long PFS for more than three years. The adverse effect was limited to proteinuria without life-threatening adverse effects, such as bleeding and bowel perforation. Due to the long-term PFS, XELOX plus bevacizumab may be considered as an excellent choice for the treatment of SBA in the metastatic setting. However, large-scale randomized clinical trials are needed to evaluate the effectiveness and the safety of this chemotherapy regimen.
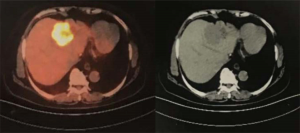
Table S1
| Gene | Gene mutation or gene fusion |
|---|---|
| AKT1 | None |
| ALK | None |
| BRAF | None |
| BRCA1 | None |
| BRCA2 | None |
| CTNNB1 | None |
| DDR2 | None |
| ESR1 | None |
| EGFR | None |
| FGFR1 | None |
| FGFR2 | None |
| FGFR3 | None |
| GNA11 | None |
| GNAQ | None |
| HER2 | None |
| HRAS | None |
| IDH1 | None |
| IDH2 | None |
| KIT | None |
| KRAS | None |
| MEK1 | None |
| MET | None |
| NF1 | None |
| NRAS | None |
| NTRK1 | None |
| PDGFRA | None |
| PIK3CA | None |
| PTEN | None |
| RET | None |
| RICTOR | None |
| ROS1 | None |
| SMAD4 | None |
| SMO | None |
| TP53 | None |
| TSC1 | None |
AKT1, AKT serine/threonine kinase 1; ALK, ALK receptor tyrosine kinase; BRAF, B-Raf proto-oncogene, serine/threonine kinase ; BRCA1, BRCA1 DNA repair associated; BRCA2, BRCA2 DNA repair associated; CTNNB1, catenin beta 1; DDR2, discoidin domain receptor tyrosine kinase 2; ESR1, estrogen receptor 1; EGFR, epidermal growth factor receptor ; FGFR1, fibroblast growth factor receptor 1; FGFR2, fibroblast growth factor receptor 2; FGFR3, fibroblast growth factor receptor 3; GNA11, G protein subunit alpha 11; GNAQ, G protein subunit alpha q; HER2, erb-b2 receptor tyrosine kinase 2; HRAS, HRas proto-oncogene, GTPase ; IDH1, isocitrate dehydrogenase (NADP(+)) 1, cytosolic; IDH2, isocitrate dehydrogenase (NADP(+)) 2, mitochondrial; KIT, KIT proto-oncogene receptor tyrosine kinase ; KRAS, KRAS proto-oncogene, GTPase; MEK1, MAP kinase/ERK kinase 1; MET, MET proto-oncogene, receptor tyrosine kinase ; NF1, neurofibromin 1; NRAS, NRAS proto-oncogene, GTPase ; NTRK1, neurotrophic receptor tyrosine kinase 1; PDGFRA, platelet derived growth factor receptor alpha; PIK3CA, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha; PTEN, phosphatase and tensin homolog ; RET, ret proto-oncogene ; RICTOR, RPTOR independent companion of MTOR complex 2; ROS1, ROS proto-oncogene 1, receptor tyrosine kinase; SMAD4, SMAD family member 4; SMO, smoothened, frizzled class receptor; TP53, tumor protein p53; TSC1, TSC complex subunit 1.
Acknowledgments
Funding: We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript. This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.06.16). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was carried out in accordance with the Institutional Ethics Committee of Shanghai Tenth People’s Hospital and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bilimoria KY, Bentrem DJ, Wayne JD, et al. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg 2009;249:63-71. [Crossref]
- Legue LM, Bernards N, Gerritse SL, et al. Trends in incidence, treatment and survival of small bowel adenocarcinomas between 1999 and 2013: a population-based study in The Netherlands. Acta Oncol 2016;55:1183-9. [Crossref]
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref]
- Aparicio T, Zaanan A, Mary F, et al. Small Bowel Adenocarcinoma. Gastroenterol Clin North Am 2016;45:447-57. [Crossref]
- Raghav K, Overman MJ. Small bowel adenocarcinomas--existing evidence and evolving paradigms. Nat Rev Clin Oncol 2013;10:534-44. [Crossref]
- Overman MJ. Rare but real: management of small bowel adenocarcinoma. Am Soc Clin Oncol Educ Book 2013;189-93. [Crossref]
- Horimatsu T, Nakayama N, Moriwaki T, et al. A phase II study of 5-fluorouracil/L-leucovorin/oxaliplatin (mFOLFOX6) in Japanese patients with metastatic or unresectable small bowel adenocarcinoma. Int J Clin Oncol 2017;22:905-12. [Crossref]
- Overman MJ, Pozadzides J, Kopetz S, et al. Immunophenotype and molecular characterisation of adenocarcinoma of the small intestine. Br J Cancer 2010;102:144-50. [Crossref]
- Gulhati P, Raghav K, Shroff RT, et al. Bevacizumab combined with capecitabine and oxaliplatin in patients with advanced adenocarcinoma of the small bowel or ampulla of vater: A single-center, open-label, phase 2 study. Cancer 2017;123:1011-7. [Crossref]
- Aydin D, Sendur MA, Kefeli U, et al. Evaluation of Bevacizumab in Advanced Small Bowel Adenocarcinoma. Clin Colorectal Cancer 2017;16:78-83. [Crossref]
- Di Francia R, De Lucia L, Di Paolo M, et al. Rational selection of predictive pharmacogenomics test for the Fluoropyrimidine/Oxaliplatin based therapy. Eur Rev Med Pharmacol Sci 2015;19:4443-54.
- Zaanan A, Dalban C, Emile JF, et al. ERCC1, XRCC1 and GSTP1 Single Nucleotide Polymorphisms and Survival of Patients with Colon Cancer Receiving Oxaliplatin-Based Adjuvant Chemotherapy. J Cancer 2014;5:425-32. [Crossref]
- Yin M, Yan J, Martinez-Balibrea E, et al. Etienne-Grimaldi MC. ERCC1 and ERCC2 polymorphisms predict clinical outcomes of oxaliplatin-based chemotherapies in gastric and colorectal cancer: a systemic review and meta-analysis. Clin Cancer Res 2011;17:1632-40. [Crossref]
- Stoehlmacher J, Ghaderi V, Iobal S, et al. A polymorphism of the XRCC1 gene predicts for response to platinum based treatment in advanced colorectal cancer. Anticancer Res 2001;21:3075-9.
- Li GY, Duan JF, Li WJ, et al. DPYD*2A/*5A/*9A and UGT1A1*6/*28 polymorphisms in Chinese colorectal cancer patients. J Cancer Res Ther 2016;12:782-6. [Crossref]
- Yeh CC, Lai CY, Chang SN, et al. Polymorphisms of MTHFR C677T and A1298C associated with survival in patients with colorectal cancer treated with 5-fluorouracil-based chemotherapy. Int J Clin Oncol 2017;22:484-93. [Crossref]
- Tsang H, Yau T, Khong PL, et al. Bevacizumab-based therapy for advanced small bowel adenocarcinoma. Gut 2008;57:1631-2. [Crossref]
- Nagaraj G, Zarbalian Y, Flora K, et al. Complete response and prolonged disease-free survival in a patient with recurrent duodenal adenocarcinoma treated with bevacizumab plus FOLFOX6. J Gastrointest Oncol 2014;5:E1-6.
- Takayoshi K, Kusaba H, Uenomachi M, et al. Suggestion of added value by bevacizumab to chemotherapy in patients with unresectable or recurrent small bowel cancer. Cancer Chemother Pharmacol 2017;80:333-42. [Crossref]
- Santini D, Fratto ME, Spoto C, et al. Cetuximab in small bowel adenocarcinoma: a new friend? Br J Cancer 2010;103:1305-author reply 1306. [Crossref]
- De Dosso S, Molinari F, Martin V, et al. Molecular characterisation and cetuximab-based treatment in a patient with refractory small bowel adenocarcinoma. Gut 2010;59:1587-8. [Crossref]
- Falcone R, Roberto M, Filetti M, et al. Anti epidermal growth factor receptor therapy in small bowel adenocarcinoma: Case report and literature review. Medicine (Baltimore) 2018;97:e9672. [Crossref]
- Zaaimi Y, Aparicio T, Laurentpuig P, et al. Advanced small bowel adenocarcinoma: Molecular characteristics and therapeutic perspectives. Clin Res Hepatol Gastroenterol 2016;40:154-60. [Crossref]
- Bläker H, Aulmann S, Helmchen B, et al. Loss of SMAD4 function in small intestinal adenocarcinomas: comparison of genetic and immunohistochemical findings. Pathol Res Pract 2004;200:1-7. [Crossref]
- Laforest A, Aparicio T, Zaanan A, et al. ERBB2 gene as a potential therapeutic target in small bowel adenocarcinoma. Eur J Cancer 2014;50:1740-16. [Crossref]