
Carcinoembryonic antigen in pleural effusion of patients with lung adenocarcinoma: a predictive marker for EGFR mutation
Introduction
Lung cancer is one of the most common malignant tumors worldwide and the 5-year survival rate was as low as 16% (1), of which non small cell lung cancer (NSCLC) accounts for approximately 80% to 85%, and lung adenocarcinoma about 40%. More than 70% of the patients present with an advanced stage at initial diagnosis, and had lost radical surgery opportunity. At present, the targeted EGFR therapy has achieved great progress. Multiple clinical studies have shown that EGFR-tyrosine kinase inhibitors (EGFR-TKIs) had a better therapeutic effect on NSCLC patients with EGFR mutations (2-9). Therefore, it is particularly important to detect EGFR mutations status before anti-tumor therapy, but EGFR gene detection sometime has some limitations because of limited tumor tissue, especially in advanced stage patients. Thus, it is necessary to uncover a safer and more reliable clinical screening method to predict EGFR mutation status.
Carcinoembryonic antigen (CEA) is widely used in clinical practice to predict treatment efficacy, prognosis, metastasis, and recurrence, etc. (5,10-12), and serum and pleural effusion (PE) are easy to obtain. However, it is still not clear whether CEA in serum and PE could be used as a biomarker to predict EGFR mutation, particularly in PE. The aim of our study is to investigate the correlation between EGFR mutation and CEA from serum and PE in advanced lung adenocarcinomas patients.
Methods
Patient selection
A total of 114 patients who were cytologically or histologically confirmed as lung adenocarcinomas with malignant pleural effusion (MPE) referred to a single institution (Jiangsu Province People’s Hospital, Nanjing, China) from November 2013 to May 2017. The following inclusion criteria were: (I) not receiving any anti-tumor therapy previously; (II) an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–2; (III) having normal hepatic, renal and hematologic functions, and no concomitant serious complications; (IV) having complete case; (V) obtaining written informed consents.
The study was approved by Medical Ethical Committee at Jiangsu Province People’s Hospital. Each patient before study-related procedures had signed the informed consent.
Detection of EGFR gene mutation and CEA
Specimens of peripheral blood and paired PE were obtained immediately before treatment. Serum and PE CEA levels were measured by immunoradiometric assay. The normal range of CEA was determined as <4.7 ng/mL. The status of EGFR mutations from exon 18 to 21 was identified using the Human EGFR Gene Mutation Detection Kit (AmoyDx, Xiamen, China), which is based on the Amplified Refractory Mutation System (ARMS).
Statistical analysis
All statistical analyses were performed using SPSS17.0 statistics software. Continuous variables were analyzed by t-tests. Fisher’s exact test or Pearson’s chi-square test procedure was used to compare categorical variables. Receiver operating characteristic (ROC) curves were constructed to determine cut-off values and evaluate the role of CEA in predicting EGFR mutations. Logistic regression analysis was employed to estimate the relationship between EGFR mutation and various factors. All reported P values were two-tailed, and P values less than 0.05 were considered statistically significant.
Results
Patient characteristics and EGFR mutation status
A total of 114 advanced adenocarcinoma patients with MPE were enrolled, and the main characteristics of the patients are shown in Table 1. There were 65 men and 49 women, with a median age of 50 (range, 29–83) years. Among all patients, EGFR gene mutations were detected in 53 of 114 cases (46.5%). The major mutation types were the exon 19 deletion (n=27, 50.9%) and the L858R point mutation in exon 21 (n=17; 32.1%). Other mutations included L861Q point mutation in exon 21 (n=1; 1.9%), exon 20 mutation (n=1; 1.9%), and exon 18 mutation (n=1; 1.9%). Additionally, 6 patients had point mutations at two sites, including 3 cases with L858R mutation in exon 21 and T790M mutation in exon 20, 2 cases with exon 18 mutation and L861Q mutation in exon 21, 1 case with exon 18 mutation and S7681 mutation in exon 20.
Table 1
| Characteristic | Patients | |
|---|---|---|
| Number | Ratio (%) | |
| Gender | ||
| Male | 65 | 57 |
| Female | 49 | 43 |
| Age | ||
| <60 | 46 | 40 |
| ≥60 | 68 | 60 |
| ECOG PS | ||
| 0 | 25 | 22 |
| 1 | 84 | 74 |
| 2 | 5 | 4 |
| Smoking history | ||
| Yes | 44 | 39 |
| No | 70 | 61 |
| Brain metastasis | ||
| Yes | 10 | 9 |
| No | 96 | 84 |
| Unknown | 8 | 7 |
| Bone metastasis | ||
| Yes | 37 | 32 |
| No | 67 | 59 |
| Unknown | 10 | 9 |
| Lymph nodes metastasis | ||
| Yes | 72 | 63 |
| No | 31 | 27 |
| Unknown | 11 | 10 |
| EGFR status | ||
| EGFR+ | 53 | 46.5 |
| L858R | 17 | 32.1 |
| 19 deletion | 27 | 50.9 |
| Exon21 L861Q | 1 | 1.9 |
| Exon18 | 1 | 1.9 |
| Exon20 | 1 | 1.9 |
| L858R/T790M | 3 | 5.7 |
| Exon18/L861Q | 2 | 3.8 |
| Exon18/S7681 | 1 | 1.9 |
| EGFR− | 61 | 53.5 |
ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor.
EGFR mutations and clinical features
We used Pearson’s chi-square test to evaluate the relationship between the incidence of EGFR gene mutations and clinical factors, the results were showed in Table 2. EGFR mutations were significantly more frequent in females than males (57.1% vs. 38.5%, P=0.048). There were no significant differences in age, smoking history, detection samples, lymph node metastasis, bone metastasis and brain metastasis between EGFR mutant and wild-type groups.
Table 2
| Characteristic | Patients | EGFR mutation status | P | |
|---|---|---|---|---|
| Positive | Negative | |||
| Gender | 0.048 | |||
| Male | 65 | 25 | 40 | |
| Female | 49 | 28 | 21 | |
| Age | 0.537 | |||
| <60 | 46 | 23 | 23 | |
| ≥60 | 68 | 30 | 38 | |
| Smoking history | 0.086 | |||
| Yes | 44 | 16 | 28 | |
| No | 70 | 37 | 33 | |
| Brain metastasis | 0.333 | |||
| Yes | 10 | 6 | 4 | |
| No | 96 | 41 | 55 | |
| Bone metastasis | 0.177 | |||
| Yes | 37 | 20 | 17 | |
| No | 67 | 27 | 40 | |
| Lymph nodes metastasis | 0.504 | |||
| Yes | 72 | 33 | 39 | |
| No | 31 | 12 | 19 | |
| Test samples | 0.440 | |||
| PE | 71 | 35 | 36 | |
| Tumor tissue | 43 | 18 | 25 | |
| Serum CEA level (ng/mL) | 0.009 | |||
| <87 | 95 | 39 | 56 | |
| ≥87 | 19 | 14 | 5 | |
| PE CEA level (ng/mL) | 0.003 | |||
| <107.2 | 56 | 18 | 38 | |
| ≥107.2 | 58 | 35 | 23 | |
CEA, carcinoembryonic antigen; EGFR, epidermal growth factor receptor.
EGFR mutations and CEA levels
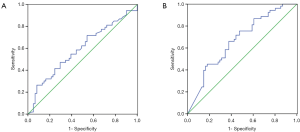
Patients harboring EGFR mutations were more likely to have higher serum and MPE CEA levels than wild-type (71.4±215 vs. 100.1±200.5 ng/mL, P=0.465; 229.3±344.8 vs. 422.2±410.9 ng/mL, P=0.008), however, there was no significant difference in serum CEA levels between two groups. An ROC curve analysis was carried out to evaluate whether the serum and PE CEA levels could predict EGFR mutation status, and find out that cut-off value of serum CEA point was 87 ng/mL, and the AUC was 0.59 (95% CI: 0.485–0.696, P=0.097) (Figure 1A). According to the selected cut-off value, the best efficacy was observed with a sensitivity (26.4%) and specificity (91.8%). ROC analysis resulted in 107.2 ng/mL as the predicting cut-off point for PE CEA, the AUC was 0.668 (95% CI: 0.569–0.767, P=0.025) (Figure 1B), and the SEN and SPE were 66% and 62.3%, respectively, with the best efficiency. The combination of gender, smoking history, serum and PE CEA level had a higher calculated AUC (0.718, 95% CI: 0.622–0.813, P=0.000) (Figure 2), and the SEN and SPE were 64.2% and 77%, respectively.
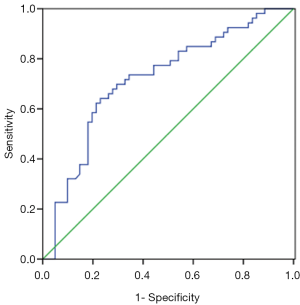
We divided the patients with advanced lung adenocarcinomas into two groups according to the cut-off value, and found that in patients with high PE and serum CEA levels (CEA ≥107.2, and ≥87 ng/mL), the EGFR mutation rate was significantly higher compared with those obtained in cases with low CEA levels (60.3% vs. 32.1%, P=0.003, and 73.7% vs. 41.1%, P=0.009).
We kept all variables with P<0.6 in a multivariate logistic analysis (Table 3), which showed an elevated odds ratio of 2.111 (95% CI: 0.738–6.035) in gender, 1.252 (95% CI: 0.416–3.769) smoking history, 2.325 (95% CI: 0.661–8.182) and 2.885 (95% CI: 1.137–7.317) serum and PE CEA level. However, the PE CEA level was confirmed as an independent factor of predicting EGFR mutations.
Table 3
| Factors | HR | 95%CI | P value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Gender | 2.111 | 0.738 | 6.035 | 0.163 |
| Age | 1.170 | 0.500 | 2.737 | 0.718 |
| Smoking history | 1.252 | 0.416 | 3.769 | 0.689 |
| Lymph nodes metastasis | 1.284 | 0.703 | 2.346 | 0.416 |
| Test samples | 1.170 | 0.463 | 2.952 | 0.740 |
| Serum CEA levels | 2.325 | 0.661 | 8.182 | 0.189 |
| PE CEA levels | 2.885 | 1.137 | 7.317 | 0.026 |
CI, confidence interval; HR, hazard ratio; CEA, carcinoembryonic antigen; PE, pleural effusion.
Discussion
EGFR mutation detection has become standard practice in determining treatment strategy of NSCLC patients; however, it is often impaired by inoperability and inadequate tumor tissue sample. Therefore, predicting EGFR gene mutation is likely to be helpful in clinical practice for patients undergoing TKI treatment. Our study retrospectively reviewed the clinical data from 114 patients with untreated advanced lung adenocarcinoma patients with MPE in Chinese population. We evaluated the association between the EGFR mutation and a comprehensive set of clinical factors, especially CEA which is widely used in clinical practice. We found that serum and PE CEA levels associated with EGFR mutations, and MPE CEA was an independent clinical factor of predicting EGFR mutation status. However, ROC analysis revealed that the AUC of MPE CEA for predicting EGFR mutation status was 0.668, and the SEN and SPE were 66% and 62.3%, respectively. Therefore, we do not depend on CEA level completely to confirm the absence or presence of EGFR mutation.
In the present study, we found the positive rate of EGFR mutations accounted for about 46.5% (53/114) in lung adenocarcinoma patients with MPE, which was consistent with several other reports reporting EGFR gene mutation rate ranging from 43% to 50% in China (13-15). However, the results of a few other studies much lower (16). The different results may be due to the selected patients. Some studies included many non-adenocarcinoma patients, which may contribute to a reduction in EGFR mutation rate. EGFR mutations were shown here predominantly occur in exon 19 (19-del) and exon 21 (L858R point mutation), while the least common mutations were exon 18 G719X, exon 20 insertion, exon 20 S768I, and exon 21 L861Q mutations. Moreover, we found that EGFR gene mutations were more often observed in female from the Pearson’s chi-square test results, which is also in accordance with previous studies (17-19).
CEA is widely used as a good tumor marker for the diagnosis, prognosis evaluation and monition, recurrence and treatment efficiency evaluation in NSCLC. Recently, several studies focused on the relationship between EGFR gene mutation status and serum tumor markers, especially CEA. Some present studies showed that serum CEA level was able to predict the EGFR mutations (20,21). However, Pan et al.’s study revealed that serum CEA may not be an ideal predictor (12). Therefore, the relationship between them was still controversial. In this study, we found that patients harboring EGFR mutations were more likely to have higher serum CEA levels than wild-type, however, there was no significant difference. In addition, ROC curve analysis revealed serum CEA is not an ideal predictor, which was consistent with Pan et al.’s study (12).
Our study is different from previous publications in evaluating the association between serum biomarkers and lung adenocarcinomas. Firstly, we focused mainly on PE CEA level and its correlation with EGFR gene status, so we selected the lung adenocarcinoma patients with MPE, and we found that a positive correlation between MPE CEA level and EGFR mutation status; in other words, EGFR gene mutation was more frequently in patients with higher PE CEA levels. The multivariate logistic analysis revealed that MPE CEA was confirmed as an independent predicting factor. Secondly, the combination of gender, smoking history, serum and MPE CEA level had a higher calculated AUC. Thirdly, we found no significant difference in the EGFR mutation rate between in tumor tissue and PE samples. The result was consistent with previous studies (22-24), suggesting that PE specimens could be used for EGFR mutation detection in advanced lung adenocarcinoma patients.
Detecting EGFR mutation in lung adenocarcinoma is necessary for determining EGFR-TKI treatment in clinic. For lung cancer patients who can tolerate surgery or tumor biopsy, it is recommended to use surgical or biopsy tissue for EGFR test, and pleural fluid for advanced lung cancer patients with PE. However, EGFR mutation detection of advanced NSCLC patients has some limitations. Firstly, the best specimen is tumor tissue from surgery, whereas 70–80% NSCLC patients have difficulties to receive radical surgery at the time of diagnosis and are unable to obtain tissue samples. Secondly, another way to obtain tissue samples is tumor biopsy which has high risk of bleeding. Moreover, there are 6.4% patients for whom obtaining enough specimen for body fluid cytology is difficult (25). Therefore, not all of the patients can undergo analysis for EGFR mutation status. Based on our data, for patients who failed to perform EGFR gene detection due to various reasons, we can predict the mutation status of EGFR gene by detecting CEA levels in serum and PE combined with clinical factors, which may ultimate benefit patients with unknown mutation status of EGFR gene from the treatment of EGFR-TKI in survival with guiding significance for clinical practice.
There are some limitations in our study. Firstly, we included patients with advanced NSCLC, and only determined the lymph nodes metastasis according to CT or integrated PET/CT findings, which might have induced a results bias. Secondly, we recorded smoking history not smoking index, which may lead to deviation of the results. Moreover, although the number of patients in our study is larger than many other similar studies, it was still small, which limits the power of multivariate analyses.
In conclusion, despite some limitations, our study indicates that MPE not serum CEA can probably serve as a marker of predicting EGFR mutation status in lung adenocarcinoma patients, and a combination of gender, smoking history, serum and MPE CEA level can play a better predictive role. However, we do not depend on CEA level completely to confirm the absence or presence of EGFR mutation. Moreover, the use of PE samples for the detection of EGFR gene mutations is highly feasible.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.06.10). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Medical Ethical Committee at Jiangsu Province People’s Hospital and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 2011;29:2866-74. [Crossref] [PubMed]
- Han JY, Park K, Kim SW, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol 2012;30:1122-8. [Crossref] [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label randomized phase 3 trial. Lancet Oncol 2010;11:121-8. [Crossref] [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [Crossref] [PubMed]
- Chen G, Feng J, Zhou C, et al. Quality of life (QoL) analyses from OPTIMAL (CTONG-0802), a phase III, randomized, open-label study of first-line erlotinib versus chemotherapy in patients with advanced EGFR mutation positive non-small-cell lung cancer (NSCLC). Ann Oncol 2013;24:1615-22. [Crossref] [PubMed]
- Costa C, Molina MA, Drozdowskyj A, et al. The impact of EGFR T790M mutations and BIM mRNA expression on outcome in patients with EGFR-mutant NSCLC treated with erlotinib or chemotherapy in the randomized phase III EURTAC trial. Clin Cancer Res 2014;20:2001-10. [Crossref] [PubMed]
- Wu YL, Zhou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol 2015;26:1883-9. [Crossref] [PubMed]
- Shi YK, Wang L, Han BH, et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol 2017;28:2443-50. [Crossref] [PubMed]
- Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 2010;11:521-9. [Crossref] [PubMed]
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353:123-32. [Crossref] [PubMed]
- Pan YQ, Shi WW, Xu DP, et al. Associations Between Epidermal Growth Factor Receptor Gene Mutation and Serum Tumor Markers in Advanced Lung Adenocarcinomas: A Retrospective Study. Chin Med Sci J 2014;29:156-61. [Crossref] [PubMed]
- Cho A, Hur J, Moon YW, et al. Correlation between EGFR mutations and serum tumor markers in lung adenocarcinoma patients. BMC Cancer 2016;16:224. [Crossref] [PubMed]
- Abdurahman A, Anwar J, Turghun A, et al. Epidermal growth factor receptor gene mutation status and its association with clinical characteristics and tumor markers in non-small-cell lung cancer patients in Northwest China. Mol Clin Oncol 2015;3:847-50. [Crossref] [PubMed]
- Gu J, Xu S, Huang L, et al. Value of combining serum carcinoembryonic antigen and PET/CT in predicting EGFR mutation in non-small cell lung cancer. J Thorac Dis 2018;10:723-31. [Crossref] [PubMed]
- Pan JB, Hou YH, Zhang GJ. Correlation between EGFR mutations and serum tumor markers in lung adenocarcinoma patients. Asian Pac J Cancer Prev 2013;14:695-700. [Crossref] [PubMed]
- Roengvoraphoj M, Tsongalis GJ, Dragnev KH, et al. Epidermal growth factor receptor tyrosine kinase inhibitors as initial therapy for non-small cell lung cancer: focus on epidermal growth factor receptor mutation testing and mutation-positive patients. Cancer Treat Rev 2013;39:839-50. [Crossref] [PubMed]
- Que D, Xiao H, Zhao B, et al. EGFR mutation status in plasma and tumor tissues in non-small cell lung cancer serves as a predictor of response to EGFR-TKI treatment. Cancer Biol Ther 2016;17:320-7. [Crossref] [PubMed]
- Zhou J, Song XB, He H, et al. Prevalence and Clinical Profile of EGFR Mutation in Non- Small-Cell Lung Carcinoma Patients in Southwest China. Asian Pac J Cancer Prev 2016;17:965-71. [Crossref] [PubMed]
- Yang ZM, Ding XP, Pen L, et al. Analysis of CEA expression and EGFR mutation status in non-small cell lung cancers. Asian Pac J Cancer Prev 2014;15:3451-5. [Crossref] [PubMed]
- Wang Z, Yang S, Lu H. Preoperative serum carcinoembryonic antigen levels are associated with histologic subtype, EGFR mutations, and ALK fusion in patients with completely resected lung adenocarcinoma. Onco Targets Ther 2017;10:3345-51. [Crossref] [PubMed]
- Liu N, Sun RZ, Du J, et al. Comparison of Epidermal Growth Factor Receptor Gene Mutations Identified Using Pleural Effusion and Primary Tumor Tissue Samples in Non-Small Cell Lung Cancer. Appl Immunohistochem Mol Morphol 2018;26:e44-51. [PubMed]
- Guan Y, Wang ZJ, Wang LQ, et al. Comparison of EGFR mutation rates in lung adenocarcinoma tissue and pleural effusion samples. Genet Mol Res 2016;15. [PubMed]
- Lin J, Gu Y, Du R, et al. Detection of EGFR mutation in supernatant, cell pellets of pleural effusion and tumor tissues from non-small cell lung cancer patients by high resolution melting analysis and sequencing. Int J Clin Exp Pathol 2014;7:8813-22. [PubMed]
- Billah S, Stewart J, Staerkel G, et al. EGFR and KRAS mutations in lung carcinoma: molecular testing by using cytology specimens. Cancer Cytopathol 2011;119:111-7. [Crossref] [PubMed]


