The efficacy of dietary Spirulina as an adjunct to chemotherapy to improve immune function and reduce myelosuppression in patients with malignant tumors
Introduction
Malignant tumors are a leading cause of death and constitute an enormous social burden worldwide (1). In general, surgical resection is currently the first choice for treatment in early-stage cancer, while chemotherapy is indispensable for advanced cancer. Due to the expanded availability of chemotherapy and the emergence of new anti-cancer drugs over the past decades, cancer patient survival rates have dramatically increased (2). However, conventional chemotherapies are often non-specific and target not only cancer cells, but also certain normal cells. As a result of this toxicity, patients may experience side-effects including granulocytopenia, febrile neutropenia, thromboembolic events, and neurosensory toxicity, necessitating skipped chemotherapy sessions, alternative chemotherapy regimens, or discontinuation of chemotherapy (3,4).
Currently, there is much focus on the use of natural products as nutritional support to improve the physical condition of patients undergoing chemotherapy. Spirulina is a genus of filamentous cyanobacteria that belongs to the Oscillatoriaceae family. Spirulina platensis and Spirulina maxima are the most widely used species and have been extensively studied in the medicine and food industry (5). Spirulina is a rich source of proteins, essential fatty acids, phenolic phytochemicals, phycobiliprotein C-phycocyanin, vitamin and minerals such as iron, copper and zinc. Beyond its rich nutritional content, it also demonstrates anti-inflammatory, oxidative stress inhibiting, and immune enhancing properties (6,7). In fact, a variety of studies have concluded that dietary Spirulina is helpful in the treatment and prevention of diabetes, diabetic nephropathy, hypercholesterolemia, and cancer (8,9).
The immunomodulatory effects are regarded as one of the most valuable properties of Spirulina. Pugh reported that oral administration of Immulina, a commercial extract from Spirulina, can reduce the severity of influenza A (H1N1) viral infection in mice model by activating innate immune cells (10). Other research has demonstrated that Spirulina can enhance innate immunity in mice and inhibit tumor growth by modulating the balance between interleukin (IL)-17/IL-23 and interferon (IFN)-γ (11). An investigation in elderly Korean participants found that Spirulina supplementation may influence the expression of inflammatory markers like IL-2 and tumor necrosis factor (TNF)-α through monocyte chemotactic protein-1 (MCP-1), suggesting that Spirulina is useful for improving immune function (12). Spirulina has also been widely applied to patients with human immunodeficiency virus (HIV). Randomized, single-blind studies showed that Spirulina could improve the nutritional status of malnourished HIV patients, leading to a significant increase in CD4+ cells and corresponding decrease in viral load (13,14). Moreover, Spirulina was reported to promote proliferation of human neural stem cells in vivo and hematopoietic stem cells in vitro through its immunomodulatory effects (15,16). Selmi et al. found that Spirulina supplementation could help to increase corpuscular hemoglobin and ameliorate anemia and immunosenescence in older patients (17). A related study concluded that Spirulina in combination with conventional iron-folic acid supplementation can bring about striking improvements in patients with nutritional anemia (18). In addition, some in vivo investigations have also reported the protective effects of dietary Spirulina on the hepatic inflammation caused by aging or toxicants (19,20). These studies revealed the anti-inflammatory, antioxidant and antihepatotoxic effects of Spirulina.
Although the immune function and antioxidant effects of Spirulina are well supported in patients with viral infections and anemia, limited studies have assessed its immunomodulatory effects in cancer patients receiving chemotherapy. To address this shortcoming, we performed a clinical trial to evaluate the effectiveness of Spirulina as an adjunct to chemotherapy to improve immune function and reduce myelosuppression in patients with malignant tumors.
Methods
Participants
From May 2017 to April 2018, 100 patients with malignant tumors undergoing chemotherapy under the supervision of the Oncology Department of Beijing Chao-Yang Hospital qualified to be included in the study. The research was carried out according to the principles set out in the Declaration of Helsinki 1964. The study protocol was approved by the Human Ethics Committee of Beijing Chao-Yang Hospital (2017-ke-313) and informed consent was obtained from all participants. Sample size and inclusion and exclusion criteria for this study were based on previous investigations with a comparable study design (21).
Inclusion criteria
Patients with an age between 18 and 70; stage II/III/IV malignant tumor; histologically confirmed malignant tumor, with or without grade I/II bone marrow suppression after receiving chemotherapy; no prior radiotherapy; adequate bone marrow reserve (HGB ≥90 g/L, absolute NEU ≥1.5×109/L and PLT ≥100×109/L) hepatic function (alanine aminotransferase (ALT) or aspartate aminotransferase (AST) ≤2.5× upper normal limits, total bilirubin ≤1.25× upper normal limits), and renal function (creatinine clearance ≥60 mL/min); an Eastern Cooperative Oncology Group performance score ≤2; generally good health and more than 3 years remaining life expectancy.
Exclusion criteria
Patients demonstrating pregnancy or lactation; evidence of central nervous system metastasis; other serious non-cancer primary diseases (e.g., cardiovascular disease, cerebrovascular system disease, hematopoietic system disease, hepatic and nephric insufficiency, psychiatric disorders, and other severe medical conditions as judged by the investigators); grade III/IV bone marrow suppression after receiving chemotherapy. Additionally, patients undergoing treatment with investigational drugs were excluded.
Treatment protocol
Patients were randomly divided into treatment and control groups according to a random number table. Spirulina was obtained from InM Wushenzhao Ecological Development Co., Ltd. (suppliers’ manufacturing lot Number: 020602B01) and the dosage included 3 capsules of 100 mg each administered 3 times daily with meals. Patients in the treated group consumed Spirulina during the first two cycles of routine chemotherapy. The control group did not consume Spirulina capsules or other drugs containing Spirulina during chemotherapy. All patients completed four cycles of personalized chemotherapy regimens.
Data collection
The characteristics of all patients were collected, including: gender, age, primary tumor, tumor metastasis, duration of chemotherapy, and treatment protocol for each cycle.
The primary effectiveness end point of this study was evaluated by detecting bone marrow suppression between the two groups in each cycle, including routine blood tests [white blood cell (WBC), neutrophilic granulocyte (NEU), hemoglobin (HGB) and platelet (PLT)], and myelosuppression-related adverse events (treatment of leukopenia, III–IV grade bone marrow suppression and alteration of chemotherapy). The lowest values of WBC, NEU, HGB and PLT were included in the analysis if there was more than one routine blood test in each cycle. The change in the results of the routine blood test across cycles was calculated using the formula: dn = baseline − Cyclen.
The secondary end-point was assessed by testing the change of immune function after all four cycles, including the level of immunoglobulins (IgA, IgG, IgM), complements (C3, C4), and CD4+ and CD8+ T lymphocytes. The change in immune function was calculated using the formula: △ = baseline − Cycle 4.
Statistical analysis
Double data entry and consistency checking were performed using EpiData 3.1. Data analyses were performed using SPSS 22.0 (IBM Corp., Armonk, NY, USA). Continuous variables such as age and blood test results are presented as mean ± standard deviation (SD). Gender and myelosuppression-related adverse events are presented as categorical variables. Significance was assessed using Chi-square test for binary categorical variables and the student’s t-test for continuous variables. P<0.05 was considered to be statistically significant.
Results
Characteristics of the study participants
A total of 100 patients that underwent four cycles of routine chemotherapy were included in the study, including 40 participants in the control group and 60 in the treatment group (Table 1). The mean age was 58.4±9.2 yrs in control group compared to 55.3±11.4 yrs in treated group. The gender proportion, types of tumor, metastasis rate, and chemotherapeutic drugs were not significantly different between two groups.
Table 1
| Characteristic | Control group | Treated group |
|---|---|---|
| Number of patients | 40 | 60 |
| Age (yrs, mean ± SD) | 58.4±9.2 | 55.3±11.4 |
| Gender (%) | ||
| Male | 17 (42.5) | 28 (46.7) |
| Female | 23 (57.5) | 32 (53.3) |
| Types of tumor (%) | ||
| Colorectal cancer | 12 (30.0) | 18 (30.0) |
| Lung cancer | 7 (17.5) | 15 (25.0) |
| Breast cancer | 4 (10.0) | 8 (13.3) |
| Pancreatic cancer | 4 (10.0) | 4 (6.7) |
| Gastric cancer | 3 (7.5) | 3 (5.0) |
| Esophageal cancer | 2 (5.0) | 2 (3.3) |
| Bladder cancer | 1 (2.5) | 2 (3.3) |
| Gallbladder carcinoma | 1 (2.5) | 1 (1.7) |
| Other type of tumor | 6 (15.0) | 7 (11.7) |
| Metastasis of tumor (%) | 23 (57.5) | 32 (53.3) |
SD, standard deviation.
Change of routine blood test after treatment
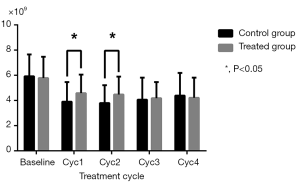
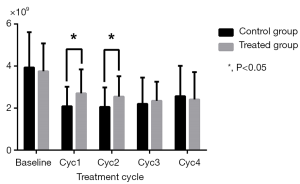
As shown in Table 2, WBC and NEU levels were similar in the two groups at baseline and in the final two cycles (P>0.05), but were increased in the treatment group compared to the control group in the Cycle1 (P=0.028 for WBC; P=0.006 for NEU) and Cycle2 (P=0.023 for WBC; P=0.013 for NEU) (Figures 1,2). HGB and PLT levels revealed no statistical differences between the two groups at baseline or following any cycle.
Table 2
| Blood test | Control group | Treated group | P value |
|---|---|---|---|
| WBC (×109) | |||
| Baseline | 5.92±1.73 | 5.78±1.69 | 0.698 |
| Cyc1 | 3.90±1.56 | 4.58±1.47 | 0.028 |
| Cyc2 | 3.80±1.41 | 4.47±1.43 | 0.023 |
| Cyc3 | 4.06±1.75 | 4.18±1.28 | 0.727 |
| Cyc4 | 4.39±1.80 | 4.21±1.60 | 0.599 |
| NEU (×109) | |||
| Baseline | 3.93±1.68 | 3.75±1.33 | 0.550 |
| Cyc1 | 2.09±0.93 | 2.70±1.14 | 0.006 |
| Cyc2 | 2.06±0.92 | 2.55±0.97 | 0.013 |
| Cyc3 | 2.20±1.25 | 2.35±0.91 | 0.495 |
| Cyc4 | 2.57±1.44 | 2.41±1.30 | 0.568 |
| HGB (g/L) | |||
| Baseline | 117±16 | 119±17 | 0.689 |
| Cyc1 | 111±15 | 116±16 | 0.098 |
| Cyc2 | 109±16 | 113±15 | 0.177 |
| Cyc3 | 109±16 | 113±15 | 0.222 |
| Cyc4 | 110±15 | 115±16 | 0.120 |
| PLT (×109) | |||
| Baseline | 227±71 | 233±80 | 0.714 |
| Cyc1 | 171±47 | 178±73 | 0.529 |
| Cyc2 | 165±42 | 181±78 | 0.171 |
| Cyc3 | 164±58 | 180±75 | 0.227 |
| Cyc4 | 172±62 | 185±77 | 0.382 |
SD, standard deviation; WBC, white blood cell; NEU, neutrophilic granulocyte; HGB, hemoglobin; PLT, platelet.
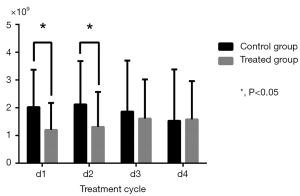
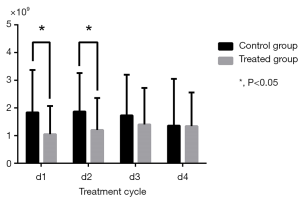
Changes in routine blood test results during cycles were calculated by comparison with baseline (Table 3). WBC and NEU levels in the control group significantly decreased in Cycle 1 (P=0.002 for WBC; P=0.006 for NEU) and Cycle2 (P=0.005 for WBC; P=0.010 for NEU) compared to the treatment group which experienced a relatively moderate decrease (Figures 3,4). HGB and PLT levels were not significantly different compared with baseline in each group and in each cycle.
Table 3
| Blood test | Control group | Treated group | P value |
|---|---|---|---|
| WBC (×109) | |||
| d1 | 2.02±1.35 | 1.20±0.97 | 0.002 |
| d2 | 2.12±1.56 | 1.31±1.26 | 0.005 |
| d3 | 1.86±1.84 | 1.61±1.41 | 0.447 |
| d4 | 1.53±1.85 | 1.58±1.38 | 0.887 |
| NEU (×109) | |||
| d1 | 1.84±1.53 | 1.05±1.02 | 0.006 |
| d2 | 1.87±1.39 | 1.20±1.16 | 0.010 |
| d3 | 1.73±1.47 | 1.40±1.32 | 0.247 |
| d4 | 1.36±1.69 | 1.34±1.22 | 0.939 |
| HGB (g/L) | |||
| d1 | 6.65±8.72 | 2.77±11.23 | 0.068 |
| d2 | 8.58±10.07 | 5.70±12.25 | 0.203 |
| d3 | 8.80±11.17 | 6.25±10.78 | 0.256 |
| d4 | 7.50±12.19 | 3.78±9.82 | 0.096 |
| PLT (×109) | |||
| d1 | 56.40±59.91 | 54.63±72.23 | 0.898 |
| d2 | 62.30±69.89 | 51.37±59.40 | 0.403 |
| d3 | 63.43±70.19 | 52.90±73.99 | 0.479 |
| d4 | 54.50±72.18 | 47.50±73.07 | 0.638 |
dn = baseline − Cyclen. SD, standard deviation; WBC, white blood cell; NEU, neutrophilic granulocyte; HGB, hemoglobin; PLT, platelet.
Change of myelosuppression after treatment
Compared to the control group, patients in the treatment group experienced fewer instances of severe myelosuppression (P=0.034) and required less alterations to their prescribed chemotherapy regimens (P=0.012) (Table 4). However, both groups had a similar leukogenic treatment rate (P=0.191).
Table 4
| Event | Control group | Treated group | P value |
|---|---|---|---|
| Leukogenic treatment (%) | 22 (55.0) | 25 (41.7) | 0.191 |
| Severe myelosuppression (%) | 17 (42.5) | 20 (33.3) | 0.034 |
| Altered treatment (%) | 22 (55.0) | 18 (30.0) | 0.012 |
Change of immunologic function after treatment
After four cycles of routine chemotherapy, the IgM level and number of CD8+ T cells increased in the treatment group, but decreased in the control group (P=0.004 for IgM; P=0.022 for CD8+ T cells) (Table 5). There were no significant differences in the levels of IgA, IgG, C3, C4 or the number of CD4+ T cells between the two groups.
Table 5
| Immune index | Control group | Treated group | P value |
|---|---|---|---|
| ΔIgA | 8.39±39.94 | 1.91±62.20 | 0.527 |
| ΔIgG | 30.60±187.07 | 10.18±253.90 | 0.664 |
| ΔIgM | 6.06±13.11 | −5.56±25.86 | 0.004 |
| ΔC3 | 1.81±17.34 | 2.83±14.26 | 0.750 |
| ΔC4 | 1.55±5.82 | 0.56±4.43 | 0.339 |
| ΔCD4+ T cells | 31.20±175.40 | −20.12±143.20 | 0.112 |
| ΔCD8+ T cells | 17.40±97.53 | −30.37±102.69 | 0.022 |
△ = baseline − Cycle 4. SD, standard deviation.
Discussion
The results of the current study support the hypothesis that Spirulina reduces myelosuppression and improves immune function after chemotherapy in patients with malignant tumors. Spirulina supplementation supported the maintenance of WBC, NEU, IgM, and CD8+ T cell population levels. Chemotherapy combined with Spirulina supplementation decreased the instances of severe myelosuppression and the need to alter treatment regimens. These results are in line with previous studies demonstrating the benefit of Spirulina supplementation in chemotherapy patients. A previous study conducted by Chen et al. first determined the effects of MB-6 (mainly composed of Spirulina) in combination with chemotherapy leucovorin/5-fluorouracil, in colorectal cancer (21). Patients receiving MB-6 had a significantly lower rate of disease progression compared to patients in the placebo group. Selmi et al. reported Spirulina was able to increase corpuscular hemoglobin steadily in senior citizens and could efficiently ameliorate anemia (17). However, our findings found no changes in HGB or PLT counts in patients receiving chemotherapy.
Most investigations into Spirulina have focused on its anti-inflammatory, antioxidant and immunomodulatory effects. However, only a few studies have attempted to investigate the mechanisms underlying these effects and our current understanding remains limited. Spirulina contains several active compounds, particularly phycocyanin and β-carotene, both of which show promising antioxidant, anti-inflammatory, and immunomodulatory activity (22). Phycocyanin is able to inhibit expression of genes regulating central factors involved in tumorigenesis, such as ornithine decarboxylase, IL-6 and pSTAT3 (23). Several studies have also attempted to elucidate the signaling pathways involved in the biological effects of Spirulina. One study found that Spirulina regulated the ERK1/2, JNK and p38 signaling pathways, resulting in anti-inflammatory and antioxidant effects (24). Others hypothesized that Spirulina might activate the JAK/STAT signaling pathways downstream of the MAPK pathway (25). However, due to the complex chemical components of Spirulina, its molecular mechanisms remain unclear and further studies are needed.
In the present study, Spirulina enhanced secretary IgM antibody response while it had limited effects on IgA and IgG. IgM, one of the main components of adaptive immune system, serves as the first line of defense in the body and is responsible for recognizing and eliminating abnormal cells and infectious particles. It exerts a cytotoxic effect on tumor cells through the complement cascade. Since IgM is the first antibody species to appear after immunological challenge, it may play a role in early detection of cancer by the immune system. Therefore, stimulating IgM production could be a promising approach by which to prevent or reduce cancer growth and guide subsequent treatment (26). Khafaga et al. reported that a specific species of Spirulina, Arthrospira platensis, exerted its effects against methotrexate-induced acute toxicity by stimulating serum immunoglobulins excluding IgM (27). The differences between this study and the current study may be related to the fluctuation of serum immunoglobulins or differences in the source material, and future studies should evaluate immunoglobulin levels in the context of these findings.
Our study also detected an increase in CD8+ T cells in patients consuming Spirulina. Immunotherapies are currently considered the most promising avenue in cancer treatment. CD8+ T cells are key effectors in anti-tumor adaptive immunity due to the fact that most tumor cells express major histocompatibility complex class I (MHC-I) (28). They are able to recognize intracellular alterations through peptides presented by MHC-I and mediate cytotoxicity efficiently. However, many studies have demonstrated that CD8+ T cells infiltrating cancer tissue are generally in dysfunctional states (CD8+ T cell exhaustion) characterized by impaired activity and proliferative ability, increased apoptosis, and reduced production of cytokines, representing a significant barrier to successful cancer elimination (29,30). Therefore, further studies should be conducted to assess whether Spirulina has can ameliorate CD8+ T cell exhaustion. Although we did not observe CD4+ T cell stimulation by Spirulina, they are known to exert an important role in tumor immune-surveillance and regulation of antigen specific immune response. Moreover, CD4+ T cells can improve the function of CD8+ T cells with high affinity for T cell receptors (TCRs) (31), so the interplay between these two populations in relation to tumor growth in patients receiving Spirulina also deserves further attention (32).
Recent studies have also argued that the combined antioxidant and immunomodulatory characteristics of Spirulina may have effects on tumor destruction and hence contribute to cancer prevention. Wang et al. reported the anti-proliferative activity of Spirulina on five cancer cell lines (HepG-2, MCF-7, SGC-7901, A549 and HT-29) and low toxicity in normal cells, suggesting that Spirulina may be a promising ingredient in food and pharmaceutical applications (33). Similarly, Liao et al. demonstrated that phycocyanin, a natural product extracted and purified from Spirulina, can effectively inhibit pancreatic cancer cell proliferation in vitro (34), and this effect is exerted by inducing apoptotic and autophagic cell death. In vivo chemopreventive effects of Spirulina have also been assessed in the literature. When Spirulina was fed to a rat breast tumor model, the incidence of breast tumors was stunningly reduced from 87% to 13% (35). However, Barakat et al. demonstrated that Spirulina lacked antitumor effects against Ehrlich carcinoma in mice, and even increased their mortality when combined with chemotherapeutic drugs like fluorouracil (36). Overall, the mechanism of Spirulina supplementation in cancer remains unclear and further studies are needed.
Limitations
The current study has several limitations. First, it was designed as a proof of concept investigation and the patient population is relatively small. Second, the control group was not prescribed a suitable placebo and the follow-up period was not long enough to evaluate additional clinical outcomes. Third, the study lacked information about the variation in response of patients with different types of neoplasm and chemotherapy regimens. Finally, the underlying direct mechanisms of Spirulina at the molecular level were not investigated in this study.
Conclusions
Our study showed the patients receiving dietary Spirulina had a lower incidence of myelosuppression and enhanced immune function. These findings suggest that Spirulina can serve as an effective and safe adjunct to chemotherapy in patients with malignant tumors.
Acknowledgments
The authors thank all participants for their participation and declare no conflict of interest.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.06.13). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Human Ethics Committee of Beijing Chao-Yang Hospital (2017-ke-313) and informed consent was obtained from all participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Marin JJ, Sanchez de Medina F, Castaño B, et al. Chemoprevention, chemotherapy, and chemoresistance in colorectal cancer. Drug Metab Rev 2012;44:148-72. [Crossref] [PubMed]
- Cassidy J, Clarke S, Díaz-Rubio E, et al. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol 2008;26:2006-12. [Crossref] [PubMed]
- Beusterien K, Grinspan J, Kuchuk I, et al. Use of conjoint analysis to assess breast cancer patient preferences for chemotherapy side effects. Oncologist 2014;19:127-34. [Crossref] [PubMed]
- Koníčková R, Vaňková K, Vaníková J, et al. Anti-cancer effects of blue-green alga Spirulina platensis, a natural source of bilirubin-like tetrapyrrolic compounds. Ann Hepatol 2014;13:273-83. [Crossref] [PubMed]
- Joventino IP, Alves HG, Neves LC, et al. The microalga Spirulina platensis presents anti-inflammatory action as well as hypoglycemic and hypolipidemic properties in diabetic rats. J Complement Integr Med 2012;9:17. [Crossref] [PubMed]
- Nielsen CH, Balachandran P, Christensen O, et al. Enhancement of natural killer cell activity in healthy subjects by Immulina(R), a Spirulina extract enriched for Braun-type lipoproteins. Planta Med 2010;76:1802-8. [Crossref] [PubMed]
- Buono S, Langellotti AL, Martello A, et al. Functional ingredients from microalgae. Food Funct 2014;5:1669-85. [Crossref] [PubMed]
- Karkos PD, Leong SC, Karkos CD, et al. Spirulina in clinical practice: evidence-based human applications. Evid Based Complement Alternat Med 2011;2011:531053. [Crossref] [PubMed]
- Pugh ND, Edwall D, Lindmark L, et al. Oral administration of a Spirulina extract enriched for Braun-type lipoproteins protects mice against influenza A (H1N1) virus infection. Phytomedicine 2015;22:271-6. [Crossref] [PubMed]
- Okuyama H, Tominaga A, Fukuoka S, et al. Spirulina lipopolysaccharides inhibit tumor growth in a Toll-like receptor 4-dependent manner by altering the cytokine milieu from interleukin-17/interleukin-23 to interferon-gamma. Oncol Rep 2017;37:684-94. [Crossref] [PubMed]
- Park HJ, Lee HS. Monocyte chemoattractant protein-1 polymorphism interaction with spirulina immunomodulatory effects in healthy Korean elderly: A 16 week, double-blind randomized clinical trial. Nutr Res Pract 2017;11:290-9. [Crossref] [PubMed]
- Azabji-Kenfack M, Dikosso SE, Loni EG, et al. Potential of Spirulina Platensis as a Nutritional Supplement in Malnourished HIV-Infected Adults in Sub-Saharan Africa: A Randomised, Single-Blind Study. Nutr Metab Insights 2011;4:29-37. [Crossref] [PubMed]
- Ngo-Matip ME, Pieme CA, Azabji-Kenfack M, et al. Impact of daily supplementation of Spirulina platensis on the immune system of naive HIV-1 patients in Cameroon: a 12-months single blind, randomized, multicenter trial. Nutr J 2015;14:70. [Crossref] [PubMed]
- Shytle DR, Tan J, Ehrhart J, et al. Effects of blue-green algae extracts on the proliferation of human adult stem cells in vitro: a preliminary study. Med Sci Monit 2010;16:BR1-5. [PubMed]
- Bachstetter AD, Jernberg J, Schlunk A, et al. Spirulina promotes stem cell genesis and protects against LPS induced declines in neural stem cell proliferation. PLoS One 2010;5:e10496. [Crossref] [PubMed]
- Selmi C, Leung PS, Fischer L, et al. The effects of Spirulina on anemia and immune function in senior citizens. Cell Mol Immunol 2011;8:248-54. [Crossref] [PubMed]
- De M, Halder A, Chakraborty T, et al. Incidence of anemia and effect of nutritional supplementation on women in rural and tribal populations of eastern and northeastern India. Hematology 2011;16:190-2. [Crossref] [PubMed]
- Neyrinck AM, Taminiau B, Walgrave H, et al. Spirulina Protects against Hepatic Inflammation in Aging: An Effect Related to the Modulation of the Gut Microbiota? Nutrients 2017; [Crossref] [PubMed]
- Al-Qahtani WH, Binobead MA. Anti-inflammatory, antioxidant and antihepatotoxic effects of Spirulina platensis against d-galactosamine induced hepatotoxicity in rats. Saudi J Biol Sci 2019;26:647-52. [Crossref] [PubMed]
- Chen WT, Yang TS, Chen HC, et al. Effectiveness of a novel herbal agent MB-6 as a potential adjunct to 5-fluoracil-based chemotherapy in colorectal cancer. Nutr Res 2014;34:585-94. [Crossref] [PubMed]
- K G MG. Supercritical CO2 extraction of functional compounds from Spirulina and their biological activity. J Food Sci Technol 2015;52:3627-33. [PubMed]
- Gupta NK, Gupta KP. Effects of C-Phycocyanin on the representative genes of tumor development in mouse skin exposed to 12-O-tetradecanoyl-phorbol-13-acetate. Environ Toxicol Pharmacol 2012;34:941-8. [Crossref] [PubMed]
- Chen HW, Yang TS, Chen MJ, et al. Purification and immunomodulating activity of C-phycocyanin from Spirulina platensis cultured using power plant flue gas. Process Biochem 2014;49:1337-44. [Crossref]
- Wu Q, Liu L, Miron A, et al. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: an overview. Arch Toxicol 2016;90:1817-40. [Crossref] [PubMed]
- Díaz-Zaragoza M, Hernández-Ávila R, Viedma-Rodríguez R, et al. Natural and adaptive IgM antibodies in the recognition of tumor-associated antigens of breast cancer Oncol Rep 2015;34:1106-14. (Review). [Crossref] [PubMed]
- Khafaga AF, El-Sayed YS. Spirulina ameliorates methotrexate hepatotoxicity via antioxidant, immune stimulation, and proinflammatory cytokines and apoptotic proteins modulation. Life Sci 2018;196:9-17. [Crossref] [PubMed]
- Protti MP, De Monte L, Di Lullo G, et al. Tumor antigen-specific CD4+ T cells in cancer immunity: from antigen identification to tumor prognosis and development of therapeutic strategies. Tissue Antigens 2014;83:237-46. [Crossref] [PubMed]
- He QF, Xu Y, Li J, et al. CD8+ T-cell exhaustion in cancer: mechanisms and new area for cancer immunotherapy. Brief Funct Genomics 2019;18:99-106. [Crossref] [PubMed]
- Prado-Garcia H, Romero-Garcia S, Aguilar-Cazares D, et al. Tumor-induced CD8+ T-cell dysfunction in lung cancer patients. Clin Dev Immunol 2012;2012:741741. [Crossref] [PubMed]
- Bos R, Marquardt KL, Cheung J, et al. Functional differences between low- and high-affinity CD8(+) T cells in the tumor environment. Oncoimmunology 2012;1:1239-47. [Crossref] [PubMed]
- Ostroumov D, Fekete-Drimusz N, Saborowski M, et al. CD4 and CD8 T lymphocyte interplay in controlling tumor growth. Cell Mol Life Sci 2018;75:689-713. [Crossref] [PubMed]
- Wang Z, Zhang X. Inhibitory effects of small molecular peptides from Spirulina (Arthrospira) platensis on cancer cell growth. Food Funct 2016;7:781-8. [Crossref] [PubMed]
- Liao G, Gao B, Gao Y, et al. Phycocyanin Inhibits Tumorigenic Potential of Pancreatic Cancer Cells: Role of Apoptosis and Autophagy. Sci Rep 2016;6:34564. [Crossref] [PubMed]
- Ouhtit A, Ismail MF, Othman A, et al. Chemoprevention of rat mammary carcinogenesis by spirulina. Am J Pathol 2014;184:296-303. [Crossref] [PubMed]
- Barakat W, Elshazly SM, Mahmoud AA, et al. Spirulina platensis Lacks Antitumor Effect against Solid Ehrlich Carcinoma in Female Mice. Adv Pharmacol Sci 2015;2015:132873. [Crossref] [PubMed]