
Multidisciplinary evaluation of locally advanced leiomyosarcoma in the lower rectum: a case report and literature review
Introduction
Leiomyosarcoma of the rectum is a rare neoplasm that accounts for fewer than 0.1% of all rectal malignancies (1). With the discovery in 1998 of CD117, a symbolic marker of gastrointestinal stromal tumor (GIST), leiomyosarcoma could be accurately diagnosed (2). Only a few studies with long-term follow-up data are available for the optimal treatment of rectal leiomyosarcoma. The prognostic factors related to tumor progression and the survival of patients are not well known. Because of the uncommon localization, we add another case of locally advanced leiomyosarcoma in the lower rectum to the literature and will review the available data in the literature.
Case presentation
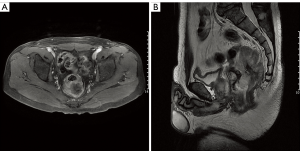
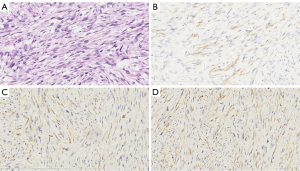
A 47-year-old man from southern China was admitted to the hospital in May 2016 and presented with anal bleeding and lower abdominal pain that sustained for two months. He had a medical history of hypertension and type II diabetes mellitus, no surgical history and a family history of genetic diseases. A digital rectal examination revealed a large rectal mass in the posterolateral wall that could not be moved, and the mass was approximately 3 cm from his anal margin. Colonoscopy showed a large submucosal lesion at the lower rectum that was partially ulcerative but mainly had normal mucosa. Contrast-enhanced computed tomography (CT) showed a large mass at the lower rectum with a diameter of 8.0 cm, and there was no evidence of lung or liver metastases. Pelvic magnetic resonance imaging (MRI) revealed an approximately 8.0 cm heterogeneous mass in the rectum invading the fascia and the perirectal tissue, with no perirectal lymph node metastasis, a positive circumferential resection margin (CRM ≤1 mm) and extramural vascular invasion (Figure 1). Serum CA 125, CEA and CA 19-9 concentrations were at normal levels. Other laboratory examinations were within normal ranges. The histological examination of the biopsy specimen showed a spindle cell neoplasm, G2, and a Ki-67 level of 30%. Immunohistochemical analysis showed position desmin, caldesmon and SMA staining, while the staining for CD117, CD34, S-100, myosin and DOG-1 were negative (Figure 2). All these results were consistent with the diagnosis of rectal leiomyosarcoma.
Because of the primary tumor size and positive CRM, this case was first discussed by an MDT. Preoperative pelvic short-course radiotherapy was indicated to reduce local recurrence. Radiotherapy with a total dose of 25 Gy was administered in 5 fractions of 5 Gy each with intensity-modulated radiotherapy. One week after radiotherapy, the patient underwent an abdominoperineal resection of the tumor by a total mesorectal excision. There was no residual macroscopic tumor tissue in surgery despite the large tumor located within a narrow pelvic space. Pathological examination of the resected specimen found that the tumor had invaded from the mucosa to the tunica adventitia, with a negative surgical margin and without vascular and perineural invasion. No lymph node metastases were found. The histological and immunohistochemical findings were the same as those with the preoperative biopsy specimen. During postoperative recovery, the patient suffered complications, including an incision infection and dysuria. The patient was discharged 22 days after the operation.
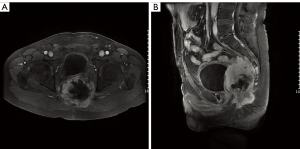
The second multidisciplinary evaluation was conducted after the resection, and adjuvant chemotherapy was suggested because of the young age of the patient and the advanced tumor, as well as his own willingness. The patient completed 4 cycles of chemotherapy (doxorubicin and ifosfamide), and the total course lasted for 3 months. After systemic therapy, regular follow-ups were performed every 3 months. Unfortunately, 13 months after the resection, the CT and MRI scans showed a local pelvic recurrence and no signs of distant metastasis (Figure 3). Subsequently, a third discussion was held by the MDT. Considering the large range of the tumor and extra radiotoxicity to part of small intestine, there was no opportunity for reoperation or more radiation therapy; thus, second-line chemotherapy was suggested. The patient completed 4 cycles of chemotherapy (gemcitabine and docetaxel), and the total course lasted nearly 4 months. CT imaging and MRI showed disease progressed after 5 months, and the patient suffered from symptoms of ureteral compression. A fourth multidisciplinary evaluation was conducted, and a renal puncture and drainage was suggested to relieve the ureteral obstruction. Radioactive particle implantation was carried out to control local progression, and the anatomical relationships between the tumor and the surrounding tissue as well as the positions and number of radioactive particles implanted were clarified using the treatment planning system (TPS). A total of 80 cores containing 125-I particles were inserted by needle into the tumor center at 20 predetermined sites. Moreover, apatinib (an oral tyrosine kinase inhibitor) was administered to gain systemic control. The patient was in a stable condition for nearly 4 months. Subsequently, the best supportive care was provided for the patient. The patient died 24 months after the diagnosis.
Discussion
Leiomyosarcomas are malignant mesenchymal neoplasms composed of cells that originate from smooth muscle lineages (3). The most common locations are the retroperitoneum, lower extremities, and the uterus. Leiomyosarcoma of the rectum is a rare neoplasm that accounts for fewer than 0.1% of all rectal malignancies. With the discovery in 1998 of CD117, a symbolic marker of gastrointestinal stromal tumor (GIST), leiomyosarcoma could be accurately diagnosed. Rectal leiomyosarcoma originates from the muscularis mucosa, muscularis propria, and walls of blood vessels. Leiomyosarcomas may grow into the mucosa or into the perirectal tissues (4). People aged 50–60 years old are susceptible to the disease. Hoshino et al. (5) reported that there were only 10 cases of rectal leiomyosarcoma in the English language literature from 1998 to 2017, including three men and seven women. The median age was 65 years, ranging from 24 to 88.
The common symptoms of rectal leiomyosarcoma are changes in bowel habits, rectal and anal pain, a pressure sensation, and anal bleeding (6). In our case, the patient was admitted with complaints of anal bleeding and lower abdominal pain. Lymphatic metastases are rare, but hematogenous metastases to distant organs and local recurrences are the primary causes of death. The initial evaluation of rectal leiomyosarcoma requires a chest and abdominal CT scan.
A pretreatment biopsy should be performed by a professional radiologist or surgeon. The biopsy should minimize contamination and complications, and the excision should be safely removed from the biopsy pathway (7). The histology of leiomyosarcoma is characterized by a dense array of spindle cells with elongated and blunt nuclei. Nuclear hyperchromasia and pleomorphism are generally characteristic patterns. The cytoplasm varies from typically brightly eosinophilic to pale. Necrosis, hemorrhage and cystic degeneration may be frequently observed (8). In the immunohistochemical examination, staining for SMA, desmin and h-caldesmon is positive in the great majority of the specimen (>70%), while staining for CD117, CD34, S-100 and DOG-1 are negative in leiomyosarcomas (9).
As reported by the American Joint Committee on Cancer (AJCC) staging system for soft tissue sarcomas, the histologic grade, tumor size, and tumor depth are three clinicopathologic risk factors for leiomyosarcomas (10). In terms of the histologic grade, the Federation Nationale des Centres de Lutte Contre le Cancer (FNCLCC) distinguishes the malignancy grade based on the three factors of differentiation, necrosis and mitotic rate (11). However, the staging of rectal leiomyosarcoma has not been determined because of its rarity. Yamamoto et al. (4) reported that tumor size (≥5 cm) was significantly associated with a poor prognosis. They also suggested that the tumor depth and necrotic area might affect the prognosis. Yeh et al. (12) stated that young age (<50 years) and high histologic tumor grade were the two independent prognostic factors for rectal leiomyosarcoma. In our case, young age, tumor size and tumor depth could be the explanatory factors for the poor prognosis.
Due to the rarity of rectal leiomyosarcoma, only a few case reports and minimal relevant data could be found in the literature. The standard treatment for rectal leiomyosarcoma remains to be resolved. Surgery is the fundamental treatment for rectal leiomyosarcomas, but surgery alone is inadequate for locally advanced tumors. It is reported that radiotherapy and chemotherapy have been proven efficacy as perioperative therapy though the efficacy was not remarkable. An MDT consisting of several specialists was essential in the management of this patient. In our department, MDT meetings for colorectal malignant tumors have been established, and the committee includes surgical oncologists, medical oncologists, radiation oncologists, pathologists and radiologists. A dedicated discussion of the relevant clinicopathological and imaging data should be held regularly.
Surgical approaches, including radical surgery, such as anterior resection, abdominoperineal resection, and local excision should aim to achieve macroscopically complete resection and microscopically minimal positive margins. Radical surgery is associated with a lower recurrence rate than wide local excision. Khalifa et al. (13) found a local recurrence rate of only 19.5% with radical resection, compared to 67.5% with local excision, but there were no differences in overall survival between the two modalities. Hoshino et al. (5) reported that a lower rectal leiomyosarcoma was moved by the hybrid method combining both the laparoscopic and transanal total mesorectal excision (TaTME) approaches because of the large tumor size and narrow pelvic space. In our case, a laparoscopic-assisted abdominoperineal resection was performed for the patient.
Traditionally, perioperative radiotherapy does not seem effective in the treatment of leiomyosarcoma. In fact, the role of postoperative radiotherapy in leiomyosarcomas of the extremities and torso has been shown to reduce local recurrence and improve local control by preserving function, although postoperative radiotherapy has not been shown to improve overall survival (14). Preoperative radiotherapy (NCT01344018) for resectable retroperitoneal sarcoma is currently under investigation and aims to improve the quality of the surgical margins (15). With the aim of maintaining local control and preserving the rectal sphincter, investigational perioperative radiotherapy for rectal leiomyosarcoma can be considered as an extension of the treatment principles for rectal cancer and limb sarcomas. In this case, because of the positive CRM ≤1 mm, radiotherapy was advised in an attempt to achieve local control. Standard TME could achieve a curative resection where there is no indication on MRI that surgery is likely to be associated with R1 resection. Finally, we chose pelvic short-course radiotherapy for a short preoperative treatment period because the use of short-course radiotherapy aims to reduce local recurrence, and downstaging/downsizing with long-course radiotherapy is not necessary. Preoperative short-course radiotherapy was carried out with a 25 Gy total dose at 5 Gy/fraction for 1 week, which was followed by immediate surgery (<10 days from the first radiation fraction). We did not choose delayed surgery because no specific chemotherapy could be used, and the disease could progress during the period of delay.
There is no accordance on the role of postoperative chemotherapy for leiomyosarcoma at present. Adjuvant chemotherapy may be an option for high-risk patients. Only two agents have been confirmed to be effective as general first-line chemotherapy, doxorubicin and ifosfamide. When leiomyosarcoma relapses or distant metastasis occurs, a poor prognosis is likely. In retrospective trials, the median progression-free survival (PFS) was approximately 6 months, and the overall survival (OS) was approximately 12 to 15 months for patients treated with doxorubicin and ifosfamide (16). According to the SARC002 study (17), the combination of a fixed-dose rate infusion of gemcitabine with docetaxel has been documented to be effective against pretreated leiomyosarcomas and leads to a median PFS and OS of 6.2 and 17.9 months, respectively. García-Del-Muro et al. (18) demonstrated the feasibility and efficacy of a fixed-dose rate of gemcitabine plus dacarbazine against pretreated leiomyosarcomas and achieved a median PFS and OS of 4.9 and 13.8 months, respectively, in a randomized phase II trial. Trabectedin has been explored and has shown a high rate of disease control (with 26–30% progression-free survival rates at 6 months), particularly in patients with leiomyosarcomas pretreated with doxorubicin and ifosfamide (19). Trabectedin is currently available in Europe and some other countries but is not yet available in China. Apatinib, an oral tyrosine kinase inhibitor that targets vascular endothelial growth factor receptor-2, was given to patients with advanced sarcoma. Zhu et al. (20) reported that the objective response rate was 33.3%, and the clinical benefit rate was 75.0%. The progression-free survival was 4.25 months, and the overall survival was 9.43 months. Compared with other histological subtypes, leiomyosarcoma did not show more significant survival benefits.
In the preoperative, postoperative, recurrent and progressive stages, four MDT discussions were conducted. Based on the accurate analysis of clinicopathologic information and a review of the literature and guidelines, the appropriate treatment was established by several specialists. Although the patient was ultimately not cured, the optimal therapeutic strategy was carried out for each disease stage, as discussed by the MDT. Through the application of a comprehensive management strategy, this patient with locally advanced leiomyosarcoma achieved a better PFS and OS than patients reported in the literature.
In conclusion, we reported a case of locally advanced rectal leiomyosarcoma treated with multidisciplinary therapy. Although the disease relapsed, and the patient survived for 24 months, MDT played a crucial role in the treatment of the rare rectal leiomyosarcoma.
Acknowledgments
Funding: This work was supported by grants from
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.06.17). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Campos FG, Leite AF, Araujo SE, et al. Anorectal leiomyomas: report of two cases with different anatomical patterns and literature review. Rev Hosp Clin Fac Med Sao Paulo 2004;59:296-301. [Crossref] [PubMed]
- Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998;279:577-80. [Crossref] [PubMed]
- Keyzer-Dekker CM, Houtkamp RG, Peterse JL, et al. Adult pelvic sarcomas: a heterogeneous collection of sarcomas? Sarcoma 2004;8:19-24. [Crossref] [PubMed]
- Yamamoto H, Handa M, Tobo T, et al. Clinicopathological features of primary leiomyosarcoma of the gastrointestinal tract following recognition of gastrointestinal stromal tumours. Histopathology 2013;63:194-207. [Crossref] [PubMed]
- Hoshino N, Hida K, Kawada K, et al. Transanal total mesorectal excision for a large leiomyosarcoma at the lower rectum: a case report and literature review. Surg Case Rep 2017;3:13. [Crossref] [PubMed]
- Zbar AP, Sokolowsky N, Sandiford N, et al. Leiomyosarcoma of the rectum. A report of two cases and review of the literature. West Indian Med J 2004;53:122-5. [PubMed]
- Merchea A, Larson DW, Hubner M, et al. The value of preoperative biopsy in the management of solid presacral tumors. Dis Colon Rectum 2013;56:756-60. [Crossref] [PubMed]
- Zambo I, Vesely K. WHO classification of tumours of soft tissue and bone 2013: the main changes compared to the 3rd edition. Cesk Patol 2014;50:64-70.
- Robin YM, Penel N, Perot G, et al. Transgelin is a novel marker of smooth muscle differentiation that improves diagnostic accuracy of leiomyosarcomas: a comparative immunohistochemical reappraisal of myogenic markers in 900 soft tissue tumors. Mod Pathol 2013;26:502-10. [Crossref] [PubMed]
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4.
- Trojani M, Contesso G, Coindre JM, et al. Soft-tissue sarcomas of adults; study of pathological prognostic variables and definition of a histopathological grading system. Int J Cancer 1984;33:37-42. [Crossref] [PubMed]
- Yeh CY, Chen HH, Tang R, et al. Surgical outcome after curative resection of rectal leiomyosarcoma. Dis Colon Rectum 2000;43:1517-21. [Crossref] [PubMed]
- Khalifa AA, Bong WL, Rao VK, et al. Leiomyosarcoma of the rectum. Report of a case and review of the literature. Dis Colon Rectum 1986;29:427-32. [Crossref] [PubMed]
- Yang JC, Chang AE, Baker AR, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol 1998;16:197-203. [Crossref] [PubMed]
- Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25:i102-12. [Crossref] [PubMed]
- Penel N, Italiano A, Isambert N, et al. Factors affecting the outcome of patients with metastatic leiomyosarcoma treated with doxorubicin-containing chemotherapy. Ann Oncol 2010;21:1361-5. [Crossref] [PubMed]
- Maki RG, Wathen JK, Patel SR, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 J Clin Oncol 2007;25:2755-63. [corrected]. [Crossref] [PubMed]
- Garcia-Del-Muro X, Lopez-Pousa A, Maurel J, et al. Randomized phase II study comparing gemcitabine plus dacarbazine versus dacarbazine alone in patients with previously treated soft tissue sarcoma: a Spanish Group for Research on Sarcomas study. J Clin Oncol 2011;29:2528-33. [Crossref] [PubMed]
- Samuels BL, Chawla S, Patel S, et al. Clinical outcomes and safety with trabectedin therapy in patients with advanced soft tissue sarcomas following failure of prior chemotherapy: results of a worldwide expanded access program study. Ann Oncol 2013;24:1703-9. [Crossref] [PubMed]
- Zhu B, Li J, Xie Q, et al. Efficacy and safety of apatinib monotherapy in advanced bone and soft tissue sarcoma: An observational study. Cancer Biol Ther 2018;19:198-204. [Crossref] [PubMed]


