
Risk of breast cancer based on thermal tomography characteristics
Introduction
Breast cancer is currently the most common malignant tumour in Chinese women (1). Since there is no good strategy for primary prevention of breast cancer, early diagnosis of breast cancer is a pivotal method to increase the survival rate and improve the prognosis of the patients (2). The development of breast cancer screening is one of the most important reasons that the mortality rate of breast cancer has been decreasing while its incidence has been increasing (1). Therefore, the development of high-efficiency screening methods is critical to improving the prognosis of the patients.
Current screening methods for breast cancer include mammography, ultrasonography and magnetic resonance imaging (MRI). Breast cancer screening must be done with mammography and ultrasonography, while MRI represents a second level instrumental examination to be reserved for those with an inherited susceptibility to breast cancer (3). Mammography is currently the preferred imaging examination method for breast cancer screening. However, it exposes patients to ionizing radiation, and its false negative rates range from 4% to 34% (4,5). In addition, it is less accurate for those with dense breast lesions or young women. Ultrasonography can be applied to patients who cannot be examined by mammography, such as young women and pregnant women, and it is convenient, safe and non-invasive, and easy to accept. However, it is less sensitive to micro-calcifications, ductal carcinoma in situ, and small invasive carcinomas, and its results are easily influenced by subjective factors (6). MRI is suitable for patients with a high risk of breast cancer due to its being highly sensitive (3,7). However, it is expensive and difficult to operate, it is not suitable for large-scale screening of populations for breast cancer.
Thermal tomography (TT) is a novel and non-invasive method for breast cancer screening and monitoring early treatment responses and has been used for early detection of breast tumours (8). It assesses breast lesions based on the angiectasis, angiogenesis and the change of temperature influenced by the metabolism of cells, which is closely related to pathological changes in the tissue (9-12). Compared to usual thermography, TT can not only detect surface temperature changes, but also generates a q-r curve to mine three-dimensional heat distribution data layer by layer based on the surface temperature distribution and reflect heat intensity varying with depth of tomography (8). In past trials we have concluded that the q-r curve for most breast cancer patients was between 30°–45°, while the q-r curve for most benign tumour patients was between 15°–30° (8). TT has the advantages of being convenient, fast, inexpensive, and objective, in addition to placing low demands on the operator and having a high sensitivity (13). As far as the large population of China is concerned, TT may be suitable for breast cancer screening. However, the correlation between the characteristics of TT and breast cancer is not clear, and there is no uniform standard for the diagnosis of breast lesions by TT (13). This may be one of the reasons why TT is not yet widely used at present.
This study is intended to clarify the correlation between TT characteristics and breast cancer, to establish the diagnostic criteria for breast lesions and define its value in distinguishing benign from malignant lesions in patients with known breast lump.
Methods
Patients
From January 2014 to July 2016, we conducted a retrospective case-control study including consecutive patients who underwent TT at Renmin Hospital of Wuhan University. The inclusion criteria were patients with a unilateral non-inflammatory single breast lesion. Patients who had undergone previous breast surgery or were pregnant were excluded. Moreover, the final diagnosis of the patient was determined by the pathological examination.
Characteristics of TT examinations
The TT system was supplied by Prof. Kaiyang Li (Department of Electronic Science and Technology, School of Physics and Technology, Wuhan University), as described previously (13). Before the examination, the patient was asked to enter the room where the temperature was 25±3 °C and had thermal source, then took off her coat and rested for 15 minutes. During this process, the patient’s breasts could not be touched. During the examination, the patient raised her hands to expose the bilateral mammary glands, and we recorded the front, left anterior, and right anterior image of the patient at 2 metres from the patient’s breast. The infrared thermal image was processed and analysed by the computer processing system. In this study, the parameters of TT were recorded, including age, q-r curve (type I: <15°, type II: 15° to 30°, type III: 30° to 45°, type IV: >45°) (8), ΔTs (surface temperature difference between the neoplastic side and the healthy side), ΔTn (nipple temperature difference between the neoplastic side and the healthy side), ΔTa (average temperature difference between the neoplastic side and the healthy side), isotherm (type I: symmetry, type II: asymmetry), vascular features (type I: normal, type II: vasodilation at the nodule, type III: discontinuity, type IV: plentiful).
Pathological diagnosis
In this study, standard pathology analysis of excised tissues was used to assess the subject’s outcomes. The excised specimens were fixed, embedded in paraffin as tissue blocks and then serially sectioned at a thickness of approximately 5 mm before being stained with haematoxylin and eosin (HE). The final pathology examinations were evaluated by an experienced pathologist for grading of pathologic tumour response of the primary breast lesion using the 2003 version of the World Health Organization (WHO) to classify the breast lesions. Three criteria were used for grading: gland formation, nuclear morphology, and mitotic figures (14).
Statistical analysis
All statistical analysis was performed with SPSS 22.0 Statistical software (IBM Corporation, Armonk, NY, USA). For TT, changes in all parameters relative to baseline were compared between the two groups and analysed for significant differences using the t-test. For determining the cut-off of ΔTs, ΔTn and ΔTn, data were analysed by receiver operating characteristic curve (ROC curve) and taking the value corresponding to the maximum value of Youden’s index (sensitivity + specificity −1) as the cut-off. For the single-predictor analysis, we used a χ2 test and Fisher’s exact test to determine which characteristics were statistically significantly associated with breast cancer. For the multiple-predictors analysis, we analysed the statistically significant predictors by using a stepwise regression, filtered the uppermost predictor, and calculated the regression coefficient per characteristic. Finally, the logic regression equation was obtained. ROC curve was used to estimate the effectiveness of the equation. The difference was significant if a two-sided P value <0.05.
Results
The study consisted of 407 cases, including 196 patients with malignant tumours with an average age of 52.34 [95% confidence interval (CI), 51.00 to 53.68] and 211 patients with benign tumours with an average age of 38.96 (95% CI, 37.38 to 40.54) (Table 1). In this series of patients undergoing breast cancer, 74.0% (145/196) of patients were diagnosed with invasive ductal carcinoma while adenoma fibroma was the primary diagnosis among patients diagnosed with benign disease, accounting for 58.8% (124/211) (Table S1). Likewise, the age distribution was wide, with 145 (74.0%) diagnosed with a malignancy at 35–60 years and 47 (24.0%) at 60 years or older. Furthermore, the incidence of malignant tumour increases with age (Table 1). As shown in Figures 1 and 2, TT showed characteristics of breast lump different from other morphological examinations such as mammography. Patients with malignant tumours had a higher breast temperature, and the mean values of ΔTs, ΔTn and ΔTa of patients with benign tumours were 0.371, 0.157 and 0.110 °C, while those of patients with malignant tumours were 0.989, 0.801, and 0.487 °C (P<0.001). The q-r curve of most patients with benign tumours was within 15–30°, while that of most patients with malignant tumours was within 30–45°. For patients with malignant tumours, their isotherm showed asymmetry, and their blood vessels were more plentiful compared to those with benign diseases.
Table 1
| Characteristics | Benign N=211 (%) | Malignancy N=196 (%) |
|---|---|---|
| Age (year) | ||
| <35 | 77 (36.5) | 4 (2.0) |
| ≥35 and <60 | 125 (59.2) | 145 (74.0) |
| ≥60 | 9 (4.3) | 47 (24.0) |
| ΔTs (°C), average (95% CI) | 0.371 (0.319, 0.423) | 0.989 (0.882, 1.097)* |
| ΔTn (°C), average (95% CI) | 0.157 (0.094, 0.220) | 0.801 (0.661, 0.941)* |
| ΔTa (°C), average (95% CI) | 0.110 (0.07, 0.149) | 0.487 (0.403, 0.571)* |
| Q-r curve | ||
| <15° | 10 (4.7) | 1 (0.5) |
| 15°–30° | 174 (82.5) | 39 (19.9) |
| 30°–45° | 26 (12.3) | 153 (78.1) |
| >45° | 1 (0.5) | 3 (1.5) |
| Isotherm | ||
| Symmetry | 93 (44.1) | 20 (10.2) |
| Asymmetry | 118 (55.9) | 176 (89.8) |
| Vascular feature | ||
| Normal | 4 (1.9) | 0 (0.0) |
| Vasodilation at the nodule | 104 (49.3) | 89 (45.4) |
| Discontinuity | 71 (33.6) | 36 (18.4) |
| Plentiful | 32 (15.2) | 71 (36.2) |
ΔTs, tumor surface temperature difference between neoplastic side and healthy one; ΔTn, nipple temperature difference between neoplastic side and healthy one; ΔTa, average temperature difference between neoplastic side and healthy one; *, two-tailed t-test P values estimate significance of the differences between the two groups (P<0.001).
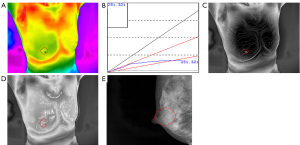
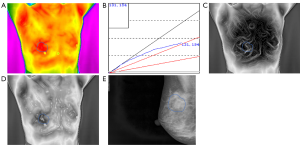
To explore the correlation between TT parameters and cancer risk, we adopted a ROC curve to assess the predictive value of ΔTs, ΔTn and ΔTa (Figure S1). We took the value corresponding to the maximum value of Youden’s index as the cut-off and found the cut-off of ΔTs, ΔTn and ΔTa were 0.65, 0.45 and 0.35 °C. Furthermore, several TT factors were significantly associated with the odds of a lesion harbouring cancer as estimated by the single-predictor model (Table 2). In terms of age, the risk of patients aged between 35 and 60 was 22.330-fold (95% CI, 7.947 to 62.748, P<0.001), and the risk of patients over 60 was 100.528-fold (95% CI, 29.313 to 344.756, P<0.001) compared with that of patients younger than 35, indicating that age had the strongest association with breast cancer. Moreover, the q-r curve also had a strong association with breast cancer, and the odds ratio (OR) of the q-r curve between 35° and 45° was 26.254 (95% CI 15.274 to 45.129, P<0.001), and the OR of the q-r curve greater than 45° was 13.385 (95% CI, 1.356 to 132.127, P=0.024). In addition, ΔTs, ΔTn, ΔTa, isotherm and vascular features were also each associated with cancer, but the magnitude of the association was smaller, with ORs ranging from 0.592 to 8.817.
Table 2
| Characteristics | OR (95% CI) | P value |
|---|---|---|
| Age (year) | ||
| <35 | 1 (reference) | |
| ≥35 and <60 | 22.330 (7.947, 62.748) | <0.001 |
| ≥60 | 100.528 (29.313, 344.756) | <0.001 |
| ΔTs (°C) | ||
| <0.65 | 1 (reference) | |
| ≥0.65 | 8.817 (5.590, 13.907) | <0. 001 |
| ΔTn (°C) | ||
| <0.45 | 1 (reference) | |
| ≥0.45 | 6.395 (4.116, 9.936) | <0.001 |
| ΔTa (°C) | ||
| <0.35 | 1 (reference) | |
| ≥0.35 | 5.313 (3.349, 8.430) | <0.001 |
| Q-r curve | ||
| <15° | 0.694 | |
| 15°–30° | 1 (reference) | |
| 30°–45° | 26.254 (15.274, 45.129) | <0.001 |
| >45° | 13.385 (1.356, 132.127) | 0.024 |
| Isotherm | ||
| Symmetry | 1 (reference) | |
| Asymmetry | 6.936 (4.056, 11.859) | <0.001 |
| Vascular feature | ||
| Normal | 0.128 | |
| Vasodilation at the nodule | 1 (reference) | |
| Discontinuity | 0.592 (0.363, 0.968) | 0.036 |
| Plentiful | 2.593 (1.566, 4.293) | <0.001 |
ΔTs, tumor surface temperature difference between neoplastic side and healthy one; ΔTn, nipple temperature difference between neoplastic side and healthy one; ΔTa, average temperature difference between neoplastic side and healthy one.
Ultimately, a total of seven characteristics were significantly associated with the risk of cancer in the multiple predictor modelling: age ≥60 years (OR =109.296, 95% CI, 21.227 to 562.763, P<0.001), age ≥35 and <60 years (OR =25.720, 95% CI, 7.129 to 92.786, P<0.001), q-r curve at an angle of 30°–45° (OR =14.895, 95% CI, 7.535 to 29.441, P<0.001), ΔTs ≥0.65 °C (OR =4.129, 95% CI, 2.030 to 8.399, P<0.001), ΔTn ≥0.45 °C (OR =2.683, 95% CI, 1.327 to 5.424, P=0.006), isotherm asymmetry (OR =2.297, 95% CI, 1.059 to 4.981, P=0.035), and vascular plentiful (OR =3.333, 95% CI, 1.455 to 7.633, P=0.004) (Table 3). The remaining characteristics were not significantly associated with cancer.
Table 3
| Characteristics | B | OR (95%CI) | P value |
|---|---|---|---|
| Age | |||
| ≥35 and <60 | 3.247 | 25.720 (7.129, 92.786) | <0.001 |
| ≥60 | 4.694 | 109.296 (21.227, 562.763) | <0.001 |
| ΔTs ≥0.65 | 1.418 | 4.129 (2.030, 8.399) | <0.001 |
| ΔTn ≥0.45 | 0.987 | 2.683 (1.327, 5.424) | 0.006 |
| Q-r curve: 30°–45° | 2.701 | 14.895 (7.535, 29.441) | <0.001 |
| Isotherm asymmetry | 0.832 | 2.297 (1.059, 4.981) | 0.035 |
| Vascular plentiful | 1.204 | 3.333 (1.455, 7.633) | 0.004 |
ΔTs, tumor surface temperature difference between neoplastic side and healthy one; ΔTn, nipple temperature difference between neoplastic side and healthy one.
Since our main aim was to establish a uniform standard for thermal imaging diagnosis of breast cancer, we constructed a regression equation based on the regression coefficients of each characteristic referring to the previous method (15). In this study, the regression equation based solely on the characteristics of TT is: P=1.418×A+0.987×B+0.832×C+2.701×D+1.204×E. The regression equation based on the characteristics of TT and age is: Q=1.418×A+0.987×B+0.832×C+2.701×D+1.204×E+3.247×F1+4.694×F2 (A: ΔTs ≥0.65 °C, B: ΔTn ≥0.45, C: isotherm asymmetry, D: q-r curve at an angle of 30°–45°, E: vascular plentiful, F1: age ≥35 and <60, F2: age ≥60). The ROC curve for the P and Q value is shown in Figure 3; the area under the curve (AUC) of the P and Q value are 0.914 and 0.943. Taking the value corresponding to the maximum value of Youden’s index as the cut-off, we found the cut-off of P and Q value were 3.4935 and 5.943. In this study, the sensitivity, specificity and accuracy of diagnosis of breast cancer by P values were 80.6%, 88.6% and 84.8%. The sensitivity, specificity and accuracy of diagnosis of breast cancer by Q values were all 86.7% (Table 4). Compared with P value, Q value has slightly lower specificity but higher sensitivity and accuracy, which is more suitable for breast cancer screening. Taken together, Q value is offered as a guideline to quantify the risk of breast cancer diagnosed by TT.
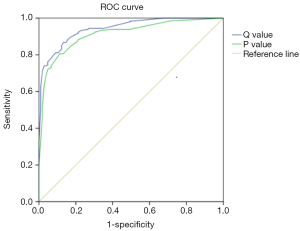
Table 4
| Variables | All patients (N=407) (%) | Benign (N=211) (%) | Malignancy (N=196) (%) |
|---|---|---|---|
| P value | |||
| Average (95% CI) | 3.09785 (2.87020 to 3.32549) | 1.45535 (1.26145 to 1.64294) | 4.86605 (4.61788 to 5.11423) |
| ≥3.4935 | 182 (44.7) | 24 (11.4) | 158 (80.6) |
| <3.4935 | 225 (55.3) | 187 (88.6) | 38 (19.4) |
| Q value | |||
| Average (95% CI) | 5.89773 (5.58904 to 6.20643) | 3.57914 (3.28829 to 3.87000) | 8.39377 (8.11667 to 8.67088) |
| ≥5.943 | 198 (48.6) | 28 (13.3) | 170 (86.7) |
| <5.943 | 209 (51.4) | 183 (86.7) | 26 (13.3) |
Discussion
Thermography has been extensively used in many medical fields, including for diabetes (16,17), influenza (18), eye diseases (19), tumours, (8) digestive disorders (20), pain diagnosing and treatment monitoring (21). And there have been many studies on thermal images of breast masses (22,23). On the basis of usual thermography, TT further obtains the relationship between heat source intensity and depth through three-dimensional reconstruction and layer-by-layer scanning. However, there is currently no uniform diagnostic criteria for the diagnosis of breast cancer by TT. In the presented study, we retrospectively analysed the results of 407 patients with non-inflammatory, unilateral, and single-breast lesions assessed by TT and established a new diagnostic standard to determine the diagnostic value of breast cancer.
Age was the most important factor. Our results indicated that patients with an older age had a higher risk of having a malignant tumour. The ages of the majority of patients with breast cancer were between 35 to 60 years old, but patients over 60 had the highest risk of getting breast cancer (OR =109.296). This conclusion is consistent with the previous research (1,24). However, the prognosis of elderly patients with breast cancer is better than that of younger patients (25,26). Therefore, age is an important consideration in the diagnosis and treatment of breast cancer.
For breast cancer, in addition to being used as a screening tool, TT can also be used to monitor tumour responses to neoadjuvant chemotherapy (27). The q-r curve is a curve that reflects heat intensity varying with depth of tomography and the difference in temperature between the lesion and the surrounding tissue. The patient’s q-r curve distribution in this trial conformed to our past conclusions (8). In this study, patients with a q-r curve of 30°–45° had the highest risk of breast cancer (OR =14.895). Q-r curves are the most important factors. Meanwhile, this might suggest in the present study that the patient was likely to suffer from breast cancer when ΔTs was greater than 0.65 °C and ΔTn was higher than 0.45 °C. Although univariate analysis showed that ΔTa was associated with breast cancer, multivariate analysis showed it was not. Many researchers have different opinions on how much the difference in breast temperature can be used as a diagnostic indicator. Head et al. (28) believed that ΔTa >0.5 °C indicated that patients were more likely to have breast cancer. In contrast, Garduño-Ramón et al. (29) believed that it was more appropriate to use 1 °C as the dividing line of ΔTs while Ng et al. (30) thought that 2 °C was suitable. This bias may be due to the differences in body temperatures. Temperature is closely related to breast disease, and an abnormal breast temperature can indicate the possibility of breast cancer in patients.
As for TT imaging, vascular and isotherm were also factors in the diagnosis of breast cancer. In most malignant tumours, tumour angiogenesis factor (TAF) stimulates tumours (31) into forming a rich vascular network in the tumour area. With the growth of the tumour, the distribution of blood vessels and the increase in the number of blood vessels are constantly increasing. Therefore, most malignant tumours have an abundant arterial blood supply, and the OR of vascular plentiful was 3.333, close to the results of ultrasonography examination (32). Although there have been few studies in the past that have mentioned the association between isotherm and breast cancer, the isotherms of the malignant tumours in this study were disorderly and dense due to the abnormal metabolism of malignant tumours, and the OR of the isotherm asymmetry was 2.297. Both vascular and isotherm abnormalities note the possibility of breast cancer.
Compared to mammography, ultrasonography and MRI (5-7,33), TT is objective and rapid and involves no contact, no radiation, and no contrast agent injection, has high repeatability and is suitable for any age group. In addition, because the changes in cell metabolism in pathological tissues occurs earlier than its functional and morphological changes, TT can detect diseases earlier (8,13). However, TT also has a few shortcomings. It is not effective for small breast cancers with a deep location. For patients with obesity or large breasts, the diagnostic results of TT are inaccurate. At the same time, TT has a poor ability to locate lesions. These shortcomings remain to be improved.
There were some limitations to this study. First, we did not classify breast cancer results by histological type. Second, there is a selection bias because we selected patients who had undergone pathological examination while some patients with an obviously low risk of breast cancer at ultrasonography did not undergo pathological examination. Third, because some patients were not examined by mammography, ultrasonography and MRI, there is no direct comparison between TT and these three modalities. Finally, the results obtained in this study are only applicable to patients with a unilateral non-inflammatory single breast lesion. Therefore, it is necessary to conduct further prospective studies to allow for longer follow-up of larger cohorts to confirm our findings.
Conclusions
Our main findings are as follows: (I) age, q-r curve, ΔTs, ΔTn, isotherm, and vascular features were independent predictors of breast cancer; (II) the Q value calculated by the logistic regression equation could be used to assess a patients’ risk of breast cancer and its accuracy was 86.7%. Based on the results of this trial, in the follow-up trials we will (I) use the equation to diagnose other patients with breast lumps to verify the results of this study; (II) use the equation to distinguish breast cancer patients sensitive to TT from insensitive patients in order to monitor the former response to neoadjuvant chemotherapy by using TT; (III) assessing the relationship between local temperature elevation of breast cancer and thermogenic proteins UCP1 and UCP2, which are highly expressed in a variety of cancer cells including breast cancer (34), make molecular probes targeting UCP1 and UCP2 to combine molecular diagnostics and TT to improve the evaluation effect of lesion size, location and depth.
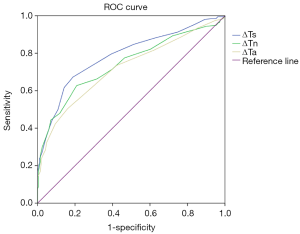
Table S1
| Types of pathology | All patients (N=407) |
|---|---|
| Malignant | 196 |
| Invasive ductal carcinoma | 145 |
| Ductal carcinoma in situ | 35 |
| Invasive lobular carcinoma | 5 |
| Mucinous adenocarcinoma | 3 |
| Invasive neuroendocrine carcinoma | 2 |
| Encapsulated papillary carcinoma | 1 |
| Invasive tubule carcinoma | 1 |
| Apocrine carcinoma | 1 |
| Squamous cell carcinoma | 1 |
| Basaloid cell carcinoma | 1 |
| Invasive cribriform carcinoma | 1 |
| Benign | 211 |
| Adenoma fibroma | 124 |
| Adenosis of mammary glands | 30 |
| Fibrocystic changes | 22 |
| Cysts | 13 |
| Intraductal papilloma | 10 |
| Hyperplasia of mammary glands | 7 |
| Lipoma | 2 |
| Phyllodes tumor of the breast | 2 |
| Mammary duct ectasia | 1 |
Acknowledgments
We thank American Journal Experts (https://secure.aje.com) for its linguistic assistance during the preparation of this manuscript.
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.06.29). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and with approval from the Ethics Committee of Renmin Hospital of Wuhan University (No. 2013-081). Informed consent was obtained from all individual participants included in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fan L, Strasser-Weippl K, Li JJ, et al. Breast cancer in China. Lancet Oncol 2014;15:e279-89. [Crossref] [PubMed]
- Tubiana M, Koscielny S. The rationale for early diagnosis of cancer--the example of breast cancer. Acta Oncol 1999;38:295-303. [Crossref] [PubMed]
- Kriege M, Brekelmans CT, Boetes C, et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med 2004;351:427-37. [Crossref] [PubMed]
- Smith-Bindman R, Chu P, Miglioretti DL, et al. Physician predictors of mammographic accuracy. J Natl Cancer Inst 2005;97:358-67. [Crossref] [PubMed]
- Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology 2002;225:165-75. [Crossref] [PubMed]
- Teh W, Wilson AR. The role of ultrasound in breast cancer screening. A consensus statement by the European Group for Breast Cancer Screening. Eur J Cancer 1998;34:449-50. [Crossref] [PubMed]
- Liberman L, Morris EA, Lee MJ, et al. Breast lesions detected on MR imaging: features and positive predictive value. AJR Am J Roentgenol 2002;179:171-8. [Crossref] [PubMed]
- Shi G, Han F, Wang L, et al. Q-r curve of thermal tomography and its clinical application on breast tumor diagnosis. Biomed Opt Express 2015;6:1109-23. [Crossref] [PubMed]
- Kandlikar SG, Perez-Raya I, Raghupathi PA, et al. Infrared imaging technology for breast cancer detection – Current status, protocols and new directions. Int J Heat Mass Transf 2017;108:2303-20. [Crossref]
- Kumar P, Kumar D, Rai KN. A numerical study on dual-phase-lag model of bio-heat transfer during hyperthermia treatment. Journal of Thermal Biology 2015;49-50:98-105. [Crossref] [PubMed]
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature 2000;407:249-57. [Crossref] [PubMed]
- Zhao Y, Bao Q, Renner A, et al. Cancer stem cells and angiogenesis. Int J Dev Biol 2011;55:477-82. [Crossref] [PubMed]
- Yao X, Wei W, Li J, et al. A comparison of mammography, ultrasonography, and far-infrared thermography with pathological results in screening and early diagnosis of breast cancer. Asian Biomedicine 2017;8:11-9. [Crossref]
- Tavassoli FA, Devilee P. editors. World Health Organization classification of tumours. Pathology and genetics of tumours of the breast and female genital organs. Lyon: IARC Press, 2003.
- Plekhanov AIu, Zhivov AV, Petrov SB, et al. Estimation of predictive prostate cancer probability with logistic regression equation. Urologiia 2007;4:81-5. [PubMed]
- Ring F. Thermal imaging today and its relevance to diabetes. J Diabetes Sci Technol 2010;4:857-62. [Crossref] [PubMed]
- Etehadtavakol M, Ng EYK, Kaabouch N. Automatic Segmentation of Thermal Images of Diabetic-at-Risk Feet Using the Snakes Algorithm. Infrared Phys Technol 2017;86:66-76. [Crossref]
- Sun G, Saga T, Shimizu T, et al. Fever screening of seasonal influenza patients using a cost-effective thermopile array with small pixels for close-range thermometry. Int J Infect Dis 2014;25:56-8. [Crossref] [PubMed]
- Tan JH, Ng EYK, Acharya UR, et al. Infrared thermography on ocular surface temperature: A review. Infrared Phys Technol 2009;52:97-108. [Crossref]
- Etehadtavakol M, Ng EYK, Emami MH. Potential of Infrared Imaging in Assessing Digestive Disorders. In: Ng E, Etehadtavakol M. editors. Application of Infrared to Biomedical Sciences. Series in BioEngineering. Singapore: Springer, 2017:1-18.
- Etehadtavakol M, Ng EYK. Potential of Thermography in Pain Diagnosing and Treatment Monitoring. In: Ng E, Etehadtavakol M. editors. Application of Infrared to Biomedical Sciences. Series in BioEngineering. Singapore: Springer, 2017:19-32.
- Etehadtavakol M, Ng EYK. Breast thermography as a potential non-contact method in the early detection of cancer: A review. J Mech Med Biol 2013;13:330001. [Crossref]
- Etehadtavakol M, Chandran V, Ng EYK, et al. Breast cancer detection from thermal images using bispectral invariant features. Int J Therm Sci 2013;69:21-36. [Crossref]
- Li H, Zheng RS, Zhang SW, et al. Incidence and mortality of female breast cancer in China, 2014. Zhonghua Zhong Liu Za Zhi 2018;40:166-71. [PubMed]
- Dobi Á, Kelemen G, Kaizer L, et al. Breast Cancer under 40 Years of Age: Increasing Number and Worse Prognosis. Pathol Oncol Res 2011;17:425-8. [Crossref] [PubMed]
- Colzani E, Liljegren A, Johansson AL, et al. Prognosis of patients with breast cancer: causes of death and effects of time since diagnosis, age, and tumor characteristics. J Clin Oncol 2011;29:4014-21. [Crossref] [PubMed]
- Wu Q, Li J, Sun S, et al. Thermal tomography for monitoring tumor response to neoadjuvant chemotherapy in women with locally advanced breast cancer. Oncotarget 2017;8:68974-83. [PubMed]
- Head JF, Lipari CA, Wang F, et al. editors. Image analysis of digitized infrared images of the breasts from a first generation infrared imaging system. Engineering in Medicine and Biology Society, 1997. Proceedings of the International Conference of the IEEE; 1997.
- Garduño-Ramón MA, Vega-Mancilla SG, Morales-Henandez LA, et al. Supportive Noninvasive Tool for the Diagnosis of Breast Cancer Using a Thermographic Camera as Sensor. Sensors (Basel) 2017; [Crossref] [PubMed]
- Ng EY, Chen Y, Ung LN. Computerized breast thermography: study of image segmentation and temperature cyclic variations. J Med Eng Technol 2001;25:12-6. [Crossref] [PubMed]
- Wang FT, Sun W, Zhang JT, et al. Cancer-associated fibroblast regulation of tumor neo-angiogenesis as a therapeutic target in cancer. Oncol Lett 2019;17:3055-65. [PubMed]
- Adler DD, Carson PL, Rubin JM, et al. Doppler ultrasound color flow imaging in the study of breast cancer: preliminary findings. Ultrasound Med Biol 1990;16:553-9. [Crossref] [PubMed]
- Berg WA, Zhang Z, Lehrer D, et al. Detection of Breast Cancer With Addition of Annual Screening Ultrasound or a Single Screening MRI to Mammography in Women With Elevated Breast Cancer Risk. JAMA 2012;307:1394-404. [Crossref] [PubMed]
- Ayyasamy V, Owens KM, Desouki MM, et al. Cellular model of Warburg effect identifies tumor promoting function of UCP2 in breast cancer and its suppression by genipin. PLoS One 2011;6:e24792. [Crossref] [PubMed]


