
The MEK inhibitors enhance the efficacy of sorafenib against hepatocellular carcinoma cells through reducing p-ERK rebound
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer and is the third leading cause of death among all cancers worldwide (1). Although surgical therapies, including resection and liver transplantation, can cure some patients in the early stages, patients are often diagnosed at an advanced stage, and these patients have a poor prognosis. Other locoregional therapies for HCC include transarterial embolization (TAE), conventional transarterial chemoembolization (cTACE), drug eluting bead (DEB), transarterial chemoembolization (DEB-TACE), and transarterial radioembolization (TARE). However, these methods have strict indications and are often accompanied by complications (2). Sorafenib was the first molecular-targeted agent that could prolong the median overall survival time for nearly 3 months compared to placebo treatment in HCC (3). However, only approximately 30% of patients can benefit from sorafenib treatment (3,4). Drug resistance limits its effectiveness, and the mechanism of sorafenib resistance has not been fully elucidated. Recent studies showed that activation of the mitogen-activated protein kinase (MAPK) signalling pathway was associated with sorafenib resistance in HCC and that the level of phosphorylated (p)-ERK was also associated with the sensitivity of HCC to sorafenib (5-8). Thus, combination therapy with sorafenib and agents that target the MAPK pathway might be an important therapeutic strategy for HCC treatment.
The RAS/RAF/MEK/ERK cascade is the major cascade reaction in the MAPK pathway. Although mutation of RAS or RAF is rare (9,10), MEK and ERK are usually activated in HCC (11-13). MEK inhibitors, including trametinib, selumetinib (AZD6244) and refametinib, have been used clinically to treat melanoma as well as several other cancers (14-16). However, the efficacy of MEK inhibitor monotherapy in the treatment of HCC is uncertain, the phase II study of monotherapy with the MEK inhibitor selumetinib in patients with advanced HCC was terminated early because of a lack of adequate antitumour activity (17). In addition, although combination therapy with sorafenib and MEK inhibitors has been widely investigated in HCC patients (18-21), experimental evidence is lacking. Thus, additional studies are needed to confirm the effectiveness of this therapeutic combination.
In the present study, we investigated whether the MEK inhibitors trametinib and selumetinib could enhance sorafenib activity in HCC cells and the mechanism underlying this effect.
Methods
Cell culture and reagents
The Bel7402 and SMMC7721 human HCC cell lines were cultured in Dulbecco’s modified Eagle’s medium containing 10% foetal calf serum (Gibco, USA) at 37 °C with 5% CO2. Sorafenib (BAY439006), trametinib (GSK1120212), and selumetinib (AZD6244) (all from Selleckchem, USA) stock solutions were prepared in DMSO (100 mM) and stored at −20 °C.
Western blot analysis
The cells were plated and allowed to adhere in complete medium overnight, followed by treatment with the indicated reagent. The cells were then lysed in RIPA buffer containing protease and phosphatase inhibitors (Calbiochem, Darmstadt, Germany). Protein lysates were harvested and centrifuged, and the supernatants were collected and quantified with a BCA protein assay (Pierce Chemical Co., USA). Equal amounts of protein sample (20 µg) were subjected to SDS-PAGE analysis and electrophoretically transferred to nitrocellulose membranes (Millipore, Bedford, USA) using a Bio-Rad semidry transfer system. Protein expression was analysed using an ODYSSEY Infrared Imaging System (LI-COR Biosciences, Lincoln, USA). The primary antibodies included phospho-MEK (Ser217/221) PARP (Cell Signaling Technology, USA), MEK1/2, ERK1/2, phospho-p44/42 MAPK (ERK) (Thr202/Tyr204), c-Myc, bax, cyclin D1 (Abcam, UK), and GAPDH (Santa Cruz, USA).
Cell proliferation and colony formation assays
The cells were plated in a 96-well plate in six replicates at a density of 3,000–5,000 cells per well. The following day, trametinib/selumetinib, sorafenib, or combinations as indicated in the figure legends were added. After 72 hours, cell proliferation was determined by a cell counting kit-8 (Dojindo Molecular Technologies, Japan) assay. The cell viability rate was calculated with the following formula: viability (%) = (average OD value of drug-treated sample/average OD value of control sample) × 100%.
For the colony formation assay, the cells were seeded onto a 35 mm dish. After overnight incubation, the cells were cultured in the absence or presence of drugs as indicated. Growth media with or without drug was replaced every 2 days. On day 7, the cells were washed three times with PBS, fixed in 4% paraformaldehyde for 15 min, stained with 0.5% crystal violet for 15 min and then imaged.
Combination index (CI) evaluation
The drug interactions between sorafenib and trametinib/selumetinib were determined by the CI value. The CI was evaluated with CompuSyn software (ComboSyn, Inc., Paramus, NJ, USA) using the method proposed by Chou et al. (22). CI values <1, =1, and >1 indicated synergistic, additive and antagonistic effects, respectively.
Statistical analysis
Statistical significance was calculated using GraphPad Prism version 5.01 and was set at *P< 0.05, **P<0.01, and ***P<0.001. Comparisons were analysed using one-way ANOVA. All experiments were performed at least three times.
Results
Sorafenib treatment caused ERK re-activation in HCC cells
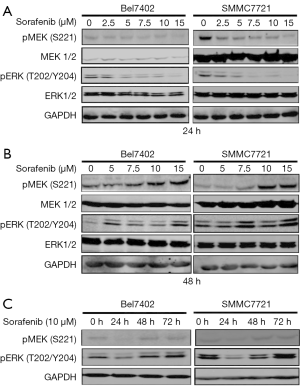
Sorafenib, which was first designed as a CRAF inhibitor, is a multikinase inhibitor, and its targets include the RAF kinases (CRAF and BRAF), VEGFR-2/3, PDGFR-β, Flt3 and c-kit (23). Some studies have indicated that the effects of sorafenib on MAPK pathways are both cell type- and context-specific (7,24). Thus, we first explored the effects of sorafenib on the MEK/ERK pathway in the cell lines used in this study (Bel7402 and SMMC7721).
The two HCC cell lines were exposed to increasing concentrations of sorafenib for 24 or 48 hours, and cell lysates were then collected for the detection of MEK/ERK activity (Figure 1A,B). The cells were also treated with 10 µM sorafenib for 24, 48, and 72 hours, and western blotting was then performed to detect the levels of phosphorylated (p)-ERK) and p-MEK (Figure 1C). Our data showed that sorafenib treatment only transiently suppressed p-MEK and p-ERK in the two HCC cell lines, and a rebound in p-ERK was observed by 48 hours, which indicated the re-activation of the MAPK pathway.
MEK inhibitors abolished the ERK activation caused by long-term sorafenib treatment in HCC cells
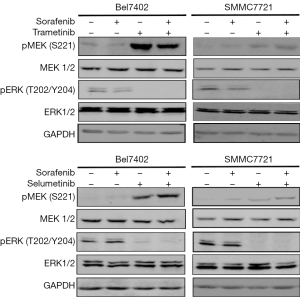
ERK is the only substrate of MEK. Therefore, we investigated whether MEK inhibitors could abrogate the re-activation of ERK caused by sorafenib. The HCC cell lines were exposed to sorafenib for 48 hours, and two selective and potent MEK inhibitors (trametinib and selumetinib) were then co-administered; after 24 hours, the whole-cell lysates were evaluated by western blotting. As shown in Figure 2, combination treatment with either trametinib or selumetinib abolished the rebound in p-ERK, while the p-MEK level was consistent with the feedback loop of MAPK pathway inhibition.
Combination treatment with sorafenib and MEK1/2 inhibitors produced synergistic suppression of HCC cell proliferation
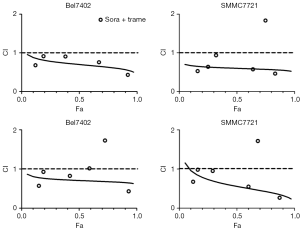
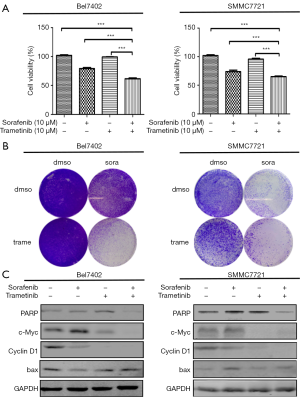
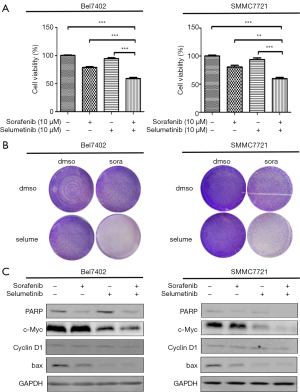
Because the MEK inhibitors selumetinib and trametinib abrogated the induction of ERK activation by sorafenib in the HCC cell lines, we accordingly hypothesized that MEK inhibitors could act synergistically with sorafenib to reduce the viability of HCC cells. The two HCC cell lines were treated with a range of concentrations of sorafenib and trametinib/selumetinib alone or combined in a fixed-ratio concentration (1:1). After 72 hours, cell viability was determined by a cell counting kit-8 assay. We first calculated the CI values using CompuSyn software (ComboSyn, Inc., Paramus, NJ, USA). According to the method proposed by Chou et al. (22), CI values <1, =1, and >1 indicated synergistic, additive and antagonistic effects, respectively. Our data showed that combination treatment with sorafenib and trametinib/selumetinib resulted in a synergistic effect in the two HCC cell lines (Figure 3). In addition, as shown in Figures 4,5, combination treatment was more effective in limiting colony formation and cell growth than sorafenib monotherapy, while trametinib or selumetinib alone had little effect on HCC cell lines. The expression levels of proteins related to proliferation (c-Myc, cyclin D1) and apoptosis (PARP, bax) were determined by western blot analysis (Figures 4C,5C). We found that trametinib/selumetinib treatment produced a significant downregulation of c-Myc and cyclin D1, while no significant alteration was observed in the level of bax following either treatment. In addition, a decrease in PARP expression was found in the combination group, but cleaved PARP was not observed.
Discussion
Although the phase II study of the MEK inhibitor selumetinib showed minimal single-agent activity in patients with advanced HCC (17), MEK is still an intriguing target for the treatment of human HCC. Some clinical trials have shown that the combination of sorafenib and MEK inhibitors presents promising activity against HCC (18,20,21). Recently, the phase I clinical study of combined treatment with the MEK inhibitor trametinib and sorafenib showed good safety and efficacy for patients with advanced HCC (19), but pre-clinical evidence is still lacking. Thus, in this study, we evaluated the effect of two MEK inhibitors (trametinib and selumetinib) combined with sorafenib on HCC cells, and furthermore, we clarified the molecular mechanism underlying this effect, ultimately providing a reasonable basis for clinical treatment.
Here, we selected two different MEK inhibitors and investigated their antitumour effects combined with sorafenib in HCC cells. The first drug was trametinib, a selective MEK inhibitor that plays a role in advanced melanoma treatment but has an unclear role in HCC. The second drug was selumetinib, and its efficacy in HCC has been demonstrated in animal models and clinical trials (18,25). By comparing the effect of these two MEK inhibitors in combination with sorafenib, we aimed to obtain new experimental evidence to support the therapeutic strategy of combining MEK inhibition with sorafenib treatment in HCC.
Notably, we observed a rebound in p-ERK signalling in the human HCC cell lines Bel7402 and SMMC7721 after long-term treatment with sorafenib. Therefore, we tested whether the MEK inhibitors trametinib and selumetinib could augment the efficacy of sorafenib through inhibiting re-activation of ERK. In addition, this hypothesis was further confirmed by western blotting, which showed that trametinib/selumetinib could downregulate the p-ERK expression induced by sorafenib, while the level of p-MEK was consistent with the feedback loop of MAPK pathway inhibition. A similar phenomenon was also observed in a previous study (26).
Furthermore, co-treatment with sorafenib and MEK inhibitors could synergistically decrease the expression of proliferation-related proteins (c-Myc and cyclin D1). However, no significant change in apoptosis-related proteins such as bax or cleaved PARP was found, but a decrease in total PARP was observed with combination treatment. The synergistic effect was demonstrated by the CI; the CI for the fixed-ratio concentration (1:1) was evaluated using CompuSyn software, and the CI values for the two cell lines were both less than 1, which indicated a synergistic effect according to the method of Chou et al. (22). However, some differences were observed between the two cell lines; the data showed that Bel7402 cells were more sensitive to the combination of an MEK inhibitor and sorafenib. The results of the colony formation assay also showed a strong antitumour effect in co-treated cells. As some RAF-inhibiting drugs could also induce paradoxical ERK pathway activation in cells with wild-type BRAF by transactivating RAF dimers (27,28), we hypothesized that a similar mechanism was responsible for the sorafenib-induced activation of ERK. Therefore, the re-activation of p-ERK caused by sorafenib in HCC cells could be reversed by treatment with the MEK inhibitors trametinib and selumetinib. In HCC, ERK overexpression and overactivation could lead to increased tumour cell proliferation, survival and invasion (11,13). Thus, dual inhibition of the MAPK pathway has a theoretical advantage in improving the activity of sorafenib in HCC treatment.
However, we also found that HCC cells were insensitive to trametinib/selumetinib as single agents. These results are consistent with data obtained from the clinical trial of selumetinib monotherapy (17). However, they are not consistent with the results of Zhou et al.’s study (29). They found that trametinib could inhibit the viability and proliferation of HCC cells, and in vivo, trametinib treatment inhibited HepG2 xenograft tumour growth and attenuated tumour invasion into surrounding tissues. Klein et al.’s study also showed that the MEK inhibitor PD184161 inhibited tumour formation in nude mice but not in tumourigenic mice (30). In addition, HCC patients rarely harbour mutations in KRAS or BRAF (9,10), while the cytotoxicity of MEK inhibitors is highly dependent on these mutations (31,32). Another important limitation may be that the effects of sorafenib and MEK inhibitors on ERK activation are both cell type- and context-specific; sorafenib has been shown to reduce the level of p-ERK in tumour cell lines that contained RAS or RAF mutations (7,33,34), and studies have also shown that BRAF-mutated cells are more sensitive to MEK inhibition (31), but the incidence of RAS and RAF gene mutations in HCC is low. Some researchers have also shown that tumours expressing higher baseline p-ERK levels were more sensitive to sorafenib (8,35). All of these observations suggest the complexity of the use of sorafenib for HCC treatment.
Conclusions
Taken together, the results of our study demonstrate that MEK inhibitors combined with sorafenib produce synergistic inhibition of the growth and survival of HCC cells. Our results supplement the accumulating evidence for this combination as a novel therapeutic strategy for HCC. Furthermore, research also shown that MEK1 shRNA transfection notably increases sorafenib-mediated lethality in lymphoma cells (36). The combination of sorafenib and AZD6244 also produced a synergistic effect in preclinical studies in renal cell carcinoma (RCC) (37) and medullary thyroid cancer (MTC) patients (38), therefore, the combination of sorafenib and MEK inhibitors may have potent antitumour activity in several cancers. In addition, further clinical investigations are urgently needed to provide more definitive evidence for the use of this combination for HCC treatment.
Acknowledgments
We would like to thank all of the participants for agreeing to participate in this research.
Funding: This work was supported by grants from
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.07.11). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The institutional ethical approval and informed consent were waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Gbolahan OB, Schacht MA, Beckley EW, et al. Locoregional and systemic therapy for hepatocellular carcinoma. J Gastrointest Oncol 2017;8:215-28. [Crossref] [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]
- Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25-34. [Crossref] [PubMed]
- Ezzoukhry Z, Louandre C, Trecherel E, et al. EGFR activation is a potential determinant of primary resistance of hepatocellular carcinoma cells to sorafenib. Int J Cancer 2012;131:2961-9. [Crossref] [PubMed]
- Rudalska R, Dauch D, Longerich T, et al. In vivo RNAi screening identifies a mechanism of sorafenib resistance in liver cancer. Nat Med 2014;20:1138-46. [Crossref] [PubMed]
- Chen Y, Liu YC, Sung YC, et al. Overcoming sorafenib evasion in hepatocellular carcinoma using CXCR4-targeted nanoparticles to co-deliver MEK-inhibitors. Sci Rep 2017;7:44123. [Crossref] [PubMed]
- Chen J, Ji T, Zhao J, et al. Sorafenib-resistant hepatocellular carcinoma stratified by phosphorylated ERK activates PD-1 immune checkpoint. Oncotarget 2016;7:41274-84. [PubMed]
- Tannapfel A, Sommerer F, Benicke M, et al. Mutations of the BRAF gene in cholangiocarcinoma but not in hepatocellular carcinoma. Gut 2003;52:706-12. [Crossref] [PubMed]
- Tada M, Omata M, Ohto M. Analysis of ras gene mutations in human hepatic malignant tumors by polymerase chain reaction and direct sequencing. Cancer Res 1990;50:1121-4. [PubMed]
- Huynh H, Nguyen TT, Chow KH, et al. Over-expression of the mitogen-activated protein kinase (MAPK) kinase (MEK)-MAPK in hepatocellular carcinoma: its role in tumor progression and apoptosis. BMC Gastroenterol 2003;3:19. [Crossref] [PubMed]
- Calvisi DF, Ladu S, Conner EA, et al. Inactivation of Ras GTPase-activating proteins promotes unrestrained activity of wild-type Ras in human liver cancer. J Hepatol 2011;54:311-9. [Crossref] [PubMed]
- Schmidt CM, McKillop IH, Cahill PA, et al. Increased MAPK expression and activity in primary human hepatocellular carcinoma. Biochem Biophys Res Commun 1997;236:54-8. [Crossref] [PubMed]
- Akinleye A, Furqan M, Mukhi N, et al. MEK and the inhibitors: from bench to bedside. J Hematol Oncol 2013;6:27. [Crossref] [PubMed]
- Zhao Y, Adjei AA. The clinical development of MEK inhibitors. Nat Rev Clin Oncol 2014;11:385-400. [Crossref] [PubMed]
- Schmieder R, Puehler F, Neuhaus R, et al. Allosteric MEK1/2 Inhibitor Refametinib (BAY 86-9766) in Combination with Sorafenib Exhibits Antitumor Activity in Preclinical Murine and Rat Models of Hepatocellular Carcinoma. Neoplasia 2013;15:1161-71. [Crossref] [PubMed]
- O'Neil BH, Goff LW, Kauh JS, et al. Phase II study of the mitogen-activated protein kinase 1/2 inhibitor selumetinib in patients with advanced hepatocellular carcinoma. J Clin Oncol 2011;29:2350-6. [Crossref] [PubMed]
- Tai WM, Yong WP, Lim C, et al. A phase Ib study of selumetinib (AZD6244, ARRY-142886) in combination with sorafenib in advanced hepatocellular carcinoma (HCC). Ann Oncol 2016;27:2210-5. [Crossref] [PubMed]
- Wang E, Kim DW, Mahipal A, et al. Phase I study of tramatinib combined with sorafenib in patients (pts) with advanced hepatocellular cancer (HCC). J Clin Oncol 2019;37:431. [Crossref]
- Lim HY, Heo J, Choi HJ, et al. A phase II study of the efficacy and safety of the combination therapy of the MEK inhibitor refametinib (BAY 86-9766) plus sorafenib for Asian patients with unresectable hepatocellular carcinoma. Clin Cancer Res 2014;20:5976-85. [Crossref] [PubMed]
- Adjei AA, Richards DA, El-Khoueiry A, et al. A Phase I Study of the Safety, Pharmacokinetics, and Pharmacodynamics of Combination Therapy with Refametinib plus Sorafenib in Patients with Advanced Cancer. Clin Cancer Res 2016;22:2368-76. [Crossref] [PubMed]
- Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 1984;22:27-55. [Crossref] [PubMed]
- Wilhelm S, Carter C, Lynch M, et al. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov 2006;5:835-44. [Crossref] [PubMed]
- Liu L, Cao Y, Chen C, et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res 2006;66:11851-8. [Crossref] [PubMed]
- Huynh H, Ngo VC, Koong HN, et al. AZD6244 enhances the anti-tumor activity of sorafenib in ectopic and orthotopic models of human hepatocellular carcinoma (HCC). J Hepatol 2010;52:79-87. [Crossref] [PubMed]
- Lin S, Hoffmann K, Xiao Z, et al. MEK inhibition induced downregulation of MRP1 and MRP3 expression in experimental hepatocellular carcinoma. Cancer Cell Int 2013;13:3. [Crossref] [PubMed]
- Poulikakos PI, Zhang C, Bollag G, et al. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature 2010;464:427-30. [Crossref] [PubMed]
- Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature 2010;464:431-5. [Crossref] [PubMed]
- Zhou X, Zhu A, Gu X, et al. Inhibition of MEK suppresses hepatocellular carcinoma growth through independent MYC and BIM regulation. Cell Oncol (Dordr) 2019;42:369-80. [Crossref] [PubMed]
- Klein PJ, Schmidt CM, Wiesenauer CA, et al. The effects of a novel MEK inhibitor PD184161 on MEK-ERK signaling and growth in human liver cancer. Neoplasia 2006;8:1-8. [Crossref] [PubMed]
- Solit DB, Garraway LA, Pratilas CA, et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature 2006;439:358-62. [Crossref] [PubMed]
- Yeh JJ, Routh ED, Rubinas T, et al. KRAS/BRAF mutation status and ERK1/2 activation as biomarkers for MEK1/2 inhibitor therapy in colorectal cancer. Mol Cancer Ther 2009;8:834-43. [Crossref] [PubMed]
- Ulivi P, Arienti C, Amadori D, et al. Role of RAF/MEK/ERK pathway, p-STAT-3 and Mcl-1 in sorafenib activity in human pancreatic cancer cell lines. J Cell Physiol 2009;220:214-21. [Crossref] [PubMed]
- Takezawa K, Okamoto I, Yonesaka K, et al. Sorafenib inhibits non-small cell lung cancer cell growth by targeting B-RAF in KRAS wild-type cells and C-RAF in KRAS mutant cells. Cancer Res 2009;69:6515-21. [Crossref] [PubMed]
- Zhang Z, Zhou X, Shen H, et al. Phosphorylated ERK is a potential predictor of sensitivity to sorafenib when treating hepatocellular carcinoma: evidence from an in vitro study. BMC Med 2009;7:41. [Crossref] [PubMed]
- Nguyen TK, Jordan N, Friedberg J, et al. Inhibition of MEK/ERK1/2 sensitizes lymphoma cells to sorafenib-induced apoptosis. Leuk Res 2010;34:379-86. [Crossref] [PubMed]
- Yuen JS, Sim MY, Sim HG, et al. Combination of the ERK inhibitor AZD6244 and low-dose sorafenib in a xenograft model of human renal cell carcinoma. Int J Oncol 2012;41:712-20. [Crossref] [PubMed]
- Koh YW, Shah MH, Agarwal K, et al. Sorafenib and Mek inhibition is synergistic in medullary thyroid carcinoma in vitro. Endocr Relat Cancer 2012;19:29-38. [Crossref] [PubMed]


