
Loss of CDH1 promotes the metastasis of hypopharyngeal squamous cell carcinoma through the STAT3-MMP-9 signaling pathway
Introduction
Tumor metastasis is the primary cause of mortality in patients with hypopharyngeal squamous cell carcinoma (HSCC) (1,2). However, despite advances in diagnostic methods and treatments, the five-year survival rate remains only at 25–40% (3), and once distant metastasis occurs, the prognoses and the survival rates of HSCC patients remain unsatisfactory (4). Therefore, a better understanding of the molecular mechanisms driving HSCC metastasis is urgently needed.
CDH1 is the prototypical member of the classical cadherin family that mediates cell–cell adhesion. Loss of CDH1 indirectly regulates gene transcription by causing the translocation of β-catenin to the nucleus (5). CDH1 loss, the hallmark of epithelial to mesenchymal transition, is a prerequisite for subsequent tumor invasion and metastasis (6,7), but the specific mechanism of CDH1 in HSCC metastasis has not been fully elucidated.
Matrix metalloproteinase (MMP)-9 is a member of the MMP family and functions as a Zn2+-dependent endopeptidase, which plays a pivotal role in tumor dissemination and invasiveness (8,9). The over-expression of MMP-9 facilitates metastasis and tumor progression by degrading the extracellular matrix (ECM), which allows tumor cells to migrate and colonize host tissues (10). MMP-9 is associated with cell growth and metastasis in several neoplasms (11-13). Importantly, the enhanced expression of MMP-9 acts the progression of head and neck squamous cell carcinoma (14). In addition, increased expression of MMP-9 is tightly linked to lymph node metastasis of laryngeal cancer (15).
Loss of CDH1 leads to the upregulation of epidermal growth factor receptor (EGFR) in head and neck cancer and in non-small cell lung cancer cells (16,17). In addition, EGFR, which is one of the receptor tyrosine kinases, can phosphorylate signal transducer and activate transcription 3 (STAT3). Once activated, STAT3 modulates the transcription of a range of targeted genes such as MMP-9, thereby influencing the proliferation, migration, and invasion of tumor cells (18,19). MMP-9 cleaves CDH1 at the cell surface, and the resulting soluble form of CDH1 promotes tumor invasion (20). However, the precise mechanism by which the downregulation of CDH1 contributes to MMP-9 upregulation remains largely unknown. In this study, we demonstrate that loss of CDH1 is closely associated with postoperative lung metastasis and is accompanied by MMP-9 upregulation. Furthermore, CDH1 enhances MMP-9 expression via STAT3 activation, which subsequently facilitates cell proliferation, invasion, and metastasis of HSCC. Therefore, our research highlights the important role of CDH1 in HSCC metastasis and might provide potential therapeutic targets for HSCC therapy.
Methods
Patients
Paired tumor and adjacent normal tissue samples were obtained from 109 patients who were undergoing radical surgery without pre-operative adjuvant treatment and who were pathologically and/or cytologically diagnosed with HSCC in Shandong Provincial Hospital affiliated to Shandong University between 2010 and 2014. The 109 enrolled patients consisted of 31 post-operative lung metastases cases, whereas the remaining 78 cases did not have lung metastases. After receiving approval from the Shandong Provincial Hospital affiliated to Shandong University Ethical Committee (No. 2017018) and signing of informed consent by patients, samples were collected and used in subsequent assays.
Immunohistochemistry (IHC)
Formalin-fixed and paraffin-embedded blocks with HSCC tissue and non-cancerous tissue were used for IHC staining as previously described (21). The rabbit monoclonal antibodies against CDH1 and MMP-9 were used as primary antibodies, and the histopathological images were obtained with an Olympus BX53 microscope. The IHC staining of CDH1 and MMP-9 expression was analyzed independently by two pathologists who were blinded to the clinical data. The sections were graded according to the amount and intensity of immunoreactivity as follows: 0 (0–5% immunopositive cells), 1 (5–25% immunopositive cells), 2 (26–50% immunopositive cells), 3 (51–80% immunopositive cells), or 4 (≥80% immunopositive cells). The intensity was scored as follows: 0 (no coloration), 1 (yellow), 2 (light brown), or 3 (dark brown). A final score was obtained by multiplying the two scores as follows: ≤1, no expression (–); 2–3, weak expression (+); 4–7, moderate expression (++); ≥8, and strong expression (+++).
Reagents
Mouse monoclonal antibodies against CDH1 (#610181) and STAT3 (#610189) were obtained from BD Transduction Laboratories. Rabbit monoclonal anti-MMP-9 (#13667) and rabbit polyclonal anti-phosphor-STAT3 (#9131) antibodies were obtained from Cell Signaling Technology. Mouse anti-β-actin monoclonal antibody (#TA-09) and the rabbit monoclonal anti-CDH1 (#ZA-0565) and anti-MMP-9 (#ZA-0562) antibodies used in the IHC assay were purchased from Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd. Purified AG490 powder (#T3434) was purchased from Sigma Aldrich.
Cell lines and cell culture
Human FaDu cell line was originally obtained from the American Type Culture Collection and was routinely tested for mycoplasma infection prior to the initiation of the experiments. The cell line was cultured in DMEM/F12 (Gibco, C11330500BT) supplemented with 10% fetal bovine serum (Ausbian, VS500T) and was maintained in a monolayer culture at 37 °C in a humidified atmosphere composed of 5% CO2 and 95% air.
Gene silencing by siRNA
All small interfering RNAs (siRNAs) were synthesized by GenePharma (Shanghai, China). Control siRNA duplexes targeted the sequence 5'-UUC UCC GAA CGU GUC ACG UTT-3'. STAT3 siRNA duplexes targeted the sequence 5'-GCA AGA UUC AGA CCC UCA ATT-3'. CDH1 siRNA #1 and #2 duplexes targeted the sequences 5'-CAG ACA AAG ACC AGG ACT A-3' and 5'-GCA CGU ACA CAG CCC UAA U-3', respectively. MMP-9 siRNA duplexes silenced the sequence 5'-CAU CAC CUA UUG GAU CCA A-3'. The FaDu cell line was transfected with the siRNAs by using the Lipofectamine® RNAiMAX Reagent according to the manufacturer’s instructions.
Western blot analysis
Whole-cell protein lysates and Western blot assays were prepared described previously (22).
Sulforhodamine B (SRB) assay
FaDu cells were transfected with siRNA in six-well plates for 24 h and further reseeded in 96-well plates with 5,000 cells/well for 48 h. Cell viability was assessed using the SRB assay as described previously (23).
CCK-8 assay
After treatment with siRNA for 24 h, the cells were reseeded in 96-well culture plates at a density of 5,000 cells/well and incubated for another 48 h. Then, 10 µL CCK-8 solution was added to each well and incubated for another 2 h at 37 °C. The absorbance was analyzed at 450 nm by utilizing a microplate reader (BioTek).
Cell migration assay
FaDu cells were transfected with siRNA for 48 h and reseeded in trans-well chambers consisting of inserts with 8 µm-pores. After further incubation for 24 h, the cell migration assay was performed as described previously (24).
Cell invasion assay
The metastatic ability of the cells was evaluated by performing invasion assay in the trans-well chambers mentioned above coated with Matrigel following the manufacturer’s protocol (Corning Incorporated).
Statistical analysis
Statistical analyses were performed using SPSS 17.0. The Mann-Whitney U-test was used to compare CDH1 and MMP-9 expression in the tumor tissues to expression in the paired adjacent normal tissues. The association between CDH1 expression and lung metastases was analyzed using Spearman’s rank correlation coefficients. Western blot, cell proliferation, migration, and invasion assays were analyzed by Student’s t-test. Statistical significance was deemed at P<0.05.
Results
Depletion of CDH1 is associated with HSCC lung metastasis and increased MMP-9 expression
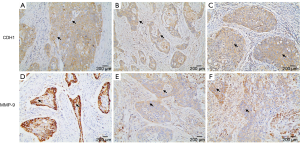
We used IHC staining to measure the expression of CDH1 and MMP-9 in 109 paired cancer and adjacent non-cancer tissues, of which 31 cases exhibited postoperative lung metastases. IHC staining showed that besides prominent localization to the membrane of tumor cells (Figure S1A), CDH1 also showed cytoplasmic expression (Figure S1B,C). However, only membrane expression was mostly found in the adjacent non-tumor cells (Figure 1A). Positive staining for CDH1 was found in 104 of 109 normal tissues, while 93 of 109 tumor tissues presented positive staining. CDH1 positive staining was significantly different between the normal (Figure 1A) and tumor tissues (Figure 1B) (P=0.012, Mann-Whitney U-test). MMP-9 expression was observed in both the adjacent non-tumor and tumor tissues, which was mainly seen as diffuse staining in the cytoplasm with occasional localization in the membrane of tumor cells (Figure S1D,E,F). The overexpression of MMP-9 protein in IHC staining was detected in 61 of 109 tumor tissues, whereas only 14 of 109 non-tumor tissues showed positive staining for MMP-9. The positive staining of MMP-9 immunoreactive protein was significantly decreased in non-tumor tissues (Figure 1C) compared with tumor tissues (Figure 1D) (P<0.001, Mann-Whitney U-test). Intriguingly, the expression of CDH1 was significantly decreased in HSCC lung metastases tissues (Figure 2A) compared with non-metastatic samples (Figure 2B). Eleven of 16 HSCC patients with no expression of CDH1 had lung metastases. The lung metastasis rate was 68.75%. Six of the 12 HSCC patients with weak (+) expression of CDH1 had lung metastases. The lung metastasis rate was 50%. For patients with moderate (++) and strong (+++) expression of CDH1, the lung metastasis rates were 24.3% (9/37) and 11.4% (5/44), respectively, which significantly differed (aP=0.000, χ2 test; Table 1). The differences between the negative expression and moderate or strong expression of CDH1 were significant (cP=0.006, dP=0.000, χ2 test, ɑ=0.008; Table 1). However, no difference was found from the comparisons between other groups. Spearman’s rank correlation coefficients showed that the expression of CDH1 was negatively correlated with HSCC lung metastases (Spearman rho =−0.431, P<0.01). Moreover, when CDH1 was downregulated, the expression of MMP-9 was correspondingly increased in the same HSCC sample (Figure 1B,D), which indicates that the expression of CDH1 might be directly associated with MMP-9 expression in HSCC.
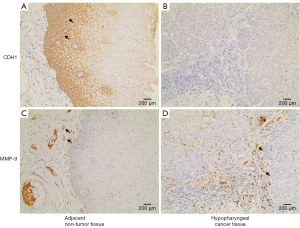
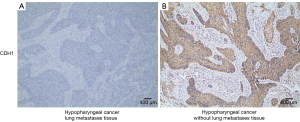
Table 1
| CDH1 expression | Non-lung metastases (%) | Lung metastases (%) | n | P |
|---|---|---|---|---|
| − | 5 (31.3) | 11 (68.7) | 16 | 0.000a |
| + | 6 (50.0) | 6 (50.0) | 12 | 0.539b |
| ++ | 28 (75.7) | 9 (24.3) | 37 | 0.006c |
| +++ | 39 (88.6) | 5 (11.4) | 44 | 0.000d |
aP=0.000 (− vs. + vs. ++ vs. +++), bP=0.539 (− vs. +), cP=0.006 (− vs. ++), dP=0.000 (− vs. +++), α=0.008. HSCC, hypopharyngeal squamous cell carcinoma.
Loss of CDH1 induces MMP-9 upregulation in FaDu cells
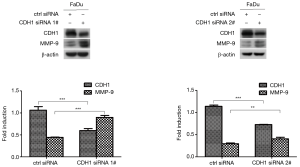
To test whether MMP-9 expression was enhanced when CDH1 was depleted in FaDu cells, we suppressed CDH1 expression with siRNA treatment in FaDu cells and measured the level of MMP-9 by Western blot assay. FaDu cells with decreased CDH1 expression showed elevated expression of MMP-9 compared with the controls (Figure 3).
Loss of CDH1 upregulated MMP-9 expression via STAT3 phosphorylation
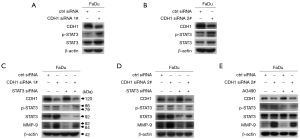
Considering that p-STAT3 contributes to MMP-9 expression (18), we further explored the effect of CDH1 depletion on the activation of STAT3. Depletion of CDH1 elicited a significant increase in p-STAT3 (Figure 4A,B). To determine whether p-STAT3 acts in MMP-9 up-regulation when CDH1 is depleted in FaDu cells, we inhibited STAT3 and CDH1 expression by siRNA transfection and found that blocking STAT3 attenuated the induction of MMP-9 caused by CDH1 depletion (Figure 4C,D). In parallel, we treated CDH1-siRNA-transfected FaDu cells with AG490 to suppress STAT3 activation and found that the pharmacological inhibition of STAT3 also prevented the induction of MMP-9 triggered by CDH1 loss (Figure 4E), which was consistent with the results of the co-transfection assay. Hence, the suppression of CDH1 leads to MMP-9 upregulation via increased phosphorylation of STAT3.
STAT3 and MMP-9 enhanced the proliferation and metastasis induced by CDH1 depletion in HSCC cells
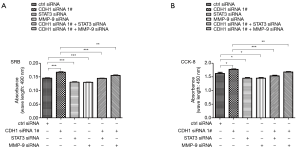
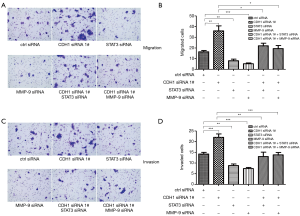
Our data showed that loss of CDH1 might contribute to tumor progression and metastasis by altering STAT3 and MMP-9 levels. To determine the roles of CDH1, STAT3, and MMP-9 in HSCC cells, a cell proliferation assay was conducted in FaDu cells. As shown in Figure 5A,B, the growth and proliferation of FaDu cells treated with CDH1 siRNA were enhanced, whereas an obvious decline was observed in the cells transfected with STAT3 or MMP-9 siRNAs. Moreover, additional knockdown of STAT3 or MMP-9 weakened the enhanced cell growth and proliferation induced by CDH1 depletion (Figure 5A,B). In addition, the metastatic potential was determined using a cell migration and invasion assay. In comparison with the control cells, knockdown of CDH1 significantly enhanced the motility of FaDu cells, whereas the migration of FaDu cells was greatly decreased when treated with STAT3 or MMP-9 siRNA. Furthermore, increased migration was observed in the CDH1-depleted cells compared with the cells co-transfected with CDH1 siRNA and either STAT3 or MMP-9 siRNA (Figure 6A,B). Finally, cell invasiveness was measured using Matrigel-coated chambers, and these results were consistent with the cell migration assay (Figure 6C,D). Hence, CDH1 depletion facilitates the proliferation, migration, and invasiveness of FaDu cells, which is accompanied by the phosphorylation of STAT3 and upregulation of MMP-9.
Discussion
HSCC has the poorest outcome among the malignant head and neck tumors, and once patients present lung metastasis, the average time from diagnosis to death is only approximately 5 months (3,25). Thus, understanding the molecular mechanisms involved in HSCC metastasis is important. Loss of CDH1 triggers active signals that support invasion and metastasis in various epithelial tumors (6), implying that it might also play an important role in HSCC lung metastases; Berx and Birchmeier et al. reported that loss of CDH1 is functionally correlated with increased incidence of metastasis (6,26). Here, we show that CDH1 downregulation is associated with HSCC lung metastases. Moreover, loss of CDH1 upregulates MMP-9 by phosphorylating STAT3 and thus contributes to the proliferation, migration, and invasiveness of FaDu cells, which might be responsible for lung metastasis and serve as an indicator for HSCC lung metastasis in clinical treatment.
Proteolytic enzymes, such as MMP-9, degrade various structural components of the ECM, thus facilitating tumor invasion into surrounding connective tissues (27). The over-expression of MMP-9 indicates metastatic potential in head and neck squamous carcinomas (28). In the present investigation, we found that reduced expression of CDH1 was concomitant with increased expression of MMP-9 in the same HSCC tissue sample. In addition, loss of CDH1 activates EGFR, which in turn phosphorylates STAT3 (16,29). Activated STAT3 upregulates the transcription of MMP-9 (18). Hence, the reduction of CDH1 affects the expression of MMP-9 through p-STAT3 in HSCC. In this study, we used two CDH1 siRNAs to transfect the FaDu cells and then measured the level of MMP-9 in FaDu cells. Our data showed that CDH1 inhibition upregulated the level of p-STAT3 and MMP-9 in FaDu cells, and silencing STAT3 attenuated the induction of MMP-9 caused by the suppression of CDH1, which indicates that CDH1 downregulation promotes MMP-9 expression in a p-STAT3-dependent manner.
Excessive cell proliferation, migration, and invasion are positively correlated with tumorigenesis and progression. Our work shows that depleting CDH1 significantly enhanced cell growth, proliferation, and invasiveness compared with the control, and silencing STAT3 and MMP-9 prevented the increased proliferation in CDH1-silenced FaDu cells. In addition, suppression of STAT3 and MMP-9 impaired the cell migration and invasion capacity caused by the loss of CDH1 in HSCC cells. Thus, the reduction of CDH1 plays a vital functional role in HSCC progression by upregulating MMP-9 via p-STAT3, hinting that p-STAT3 and MMP-9 might serve as crucial targets for the clinical treatment of HSCC.
Conclusions
In summary, we showed that CDH1 is a powerful indicator for HSCC lung metastasis, loss of CDH1 elevates MMP-9 expression by activating STAT3. Subsequently, the proliferative, invasive, and metastatic capacity of FaDu cells are promoted, which might contribute to the malignant development and lung metastases of HSCC. Taken together, our study might provide novel therapeutic targets for metastatic HSCC and introduce new avenues for the further development of clinical treatments.
Acknowledgments
We thank Mr. Martin J. Booth, Dr. Haiqiong Shang, Dr. Jingcheng Gu, and Dr. Ying Cui for assistance in writing the manuscript.
Funding: This work was supported by grants from
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.07.51). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Shandong Provincial Hospital Affiliated to Shandong University Ethical Committee (No. 2017018) and all patients signed informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hui AB, Bruce JP, Alajez NM, et al. Significance of dysregulated metadherin and microRNA-375 in head and neck cancer. Clin Cancer Res 2011;17:7539-50. [Crossref] [PubMed]
- Loberg RD, Bradley DA, Tomlins SA, et al. The lethal phenotype of cancer: the molecular basis of death due to malignancy. CA Cancer J Clin 2007;57:225-41. [Crossref] [PubMed]
- Wycliffe ND, Grover RS, Kim PD, et al. Hypopharyngeal cancer. Top Magn Reson Imaging 2007;18:243-58. [Crossref] [PubMed]
- Garavello W, Ciardo A, Spreafico R, et al. Risk factors for distant metastases in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg 2006;132:762-6. [Crossref] [PubMed]
- Yasmeen A, Bismar TA, Al Moustafa AE. ErbB receptors and epithelial-cadherin-catenin complex in human carcinomas. Future Oncol 2006;2:765-81. [Crossref] [PubMed]
- Birchmeier W, Behrens J. Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochimica et Biophysica Acta 1994;1198:11-26. [PubMed]
- Guarino M, Rubino B, Ballabio G. The role of epithelial-mesenchymal transition in cancer pathology. Pathology 2007;39:305-18. [Crossref] [PubMed]
- Wieczorek E, Jablonska E, Wasowicz W, et al. Matrix metalloproteinases and genetic mouse models in cancer research: a mini-review. Tumour Biol 2015;36:163-75. [Crossref] [PubMed]
- Foda HD, Zucker S. Matrix metalloproteinases in cancer invasion, metastasis and angiogenesis. Drug Discov Today 2001;6:478-82. [Crossref] [PubMed]
- Klein G, Vellenga E, Fraaije MW, et al. The possible role of matrix metalloproteinase (MMP)-2 and MMP-9 in cancer, e.g. acute leukemia. Crit Rev Oncol Hematol 2004;50:87-100. [Crossref] [PubMed]
- Vihinen P, Kahari VM. Matrix metalloproteinases in cancer: prognostic markers and therapeutic targets. Int J Cancer 2002;99:157-66. [Crossref] [PubMed]
- Nelson AR, Fingleton B, Rothenberg ML, et al. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol 2000;18:1135-49. [Crossref] [PubMed]
- Westermarck J, Kahari VM. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J 1999;13:781-92. [Crossref] [PubMed]
- Ruokolainen H, Paakko P, Turpeenniemi-Hujanen T. Expression of matrix metalloproteinase-9 in head and neck squamous cell carcinoma: a potential marker for prognosis. Clin Cancer Res 2004;10:3110-16. [Crossref] [PubMed]
- Xie M, Sun Y, Li Y. Expression of matrix metalloproteinases in supraglottic carcinoma and its clinical implication for estimating lymph node metastases. Laryngoscope 2004;114:2243-8. [Crossref] [PubMed]
- Wang D, Su L, Huang D, et al. Downregulation of E-Cadherin enhances proliferation of head and neck cancer through transcriptional regulation of EGFR. Mol Cancer 2011;10:116. [Crossref] [PubMed]
- Liu X, Su L, Liu X. Loss of CDH1 up-regulates epidermal growth factor receptor via phosphorylation of YBX1 in non-small cell lung cancer cells. FEBS Lett 2013;587:3995-4000. [Crossref] [PubMed]
- Liu X, Lv Z, Zou J, et al. Elevated AEG-1 expression in macrophages promotes hypopharyngeal cancer invasion through the STAT3-MMP-9 signaling pathway. Oncotarget 2016;7:77244-56. [PubMed]
- Yuan J, Zhang F, Niu R. Multiple regulation pathways and pivotal biological functions of STAT3 in cancer. Sci Rep 2015;5:17663. [Crossref] [PubMed]
- Nawrocki-Raby B, Gilles C, Polette M, et al. Upregulation of MMPs by soluble E-cadherin in human lung tumor cells. Int J Cancer 2003;105:790-5. [Crossref] [PubMed]
- Wang CL, Wang CI, Liao PC, et al. Discovery of retinoblastoma-associated binding protein 46 as a novel prognostic marker for distant metastasis in nonsmall cell lung cancer by combined analysis of cancer cell secretome and pleural effusion proteome. J Proteome Res 2009;8:4428-40. [Crossref] [PubMed]
- Liu X, Yue P, Zhou Z, et al. Death receptor regulation and celecoxib-induced apoptosis in human lung cancer cells. J Natl Cancer Inst 2004;96:1769-80. [Crossref] [PubMed]
- Su L, Liu G, Hao X, et al. Death receptor 5 and cellular FLICE-inhibitory protein regulate pemetrexed-induced apoptosis in human lung cancer cells. Eur J Cancer 2011;47:2471-8. [Crossref] [PubMed]
- Cowden Dahl KD, Zeineldin R, Hudson LG. PEA3 is necessary for optimal epidermal growth factor receptor-stimulated matrix metalloproteinase expression and invasion of ovarian tumor cells. Mol Cancer Res 2007;5:413-21. [Crossref] [PubMed]
- Alvi A, Johnson JT. Development of distant metastasis after treatment of advanced-stage head and neck cancer. Head Neck 1997;19:500-5. [Crossref] [PubMed]
- Berx G, van Roy F. Involvement of members of the cadherin superfamily in cancer. Cold Spring Harb Perspect Biol 2009;1:a003129. [Crossref] [PubMed]
- Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science 2002;295:2387-92. [Crossref] [PubMed]
- Roomi MW, Kalinovsky T, Roomi NW, et al. In vitro and in vivo inhibition of human Fanconi anemia head and neck squamous carcinoma by a phytonutrient combination. Int J Oncol 2015;46:2261-6. [Crossref] [PubMed]
- Abdelhamed S, Ogura K, Yokoyama S, et al. AKT-STAT3 Pathway as a Downstream Target of EGFR Signaling to Regulate PD-L1 Expression on NSCLC cells. J Cancer 2016;7:1579-86. [Crossref] [PubMed]


