Short-term clinical outcomes of enteral nutrition versus parenteral nutrition after surgery for pancreatic cancer: a meta-analysis
Introduction
Pancreatic cancer is now the seventh leading cause of cancer death in both sexes together, and it leads to an estimated 432,242 deaths globally in 2018 (1-3). Because of its poor prognosis, with almost as many deaths as cases, it has been projected that pancreatic cancer will emerge as the third leading cause of cancer death in the future (1). Despite development in detection techniques and management of pancreatic cancer, the estimated 5-year survival rate for pancreatic cancer is less than 5% (4,5).
Although pancreaticoduodenectomy (PD) has become a standard operation for patients with periampullary tumors and pancreatic head cancer, postoperative complications including malabsorption still threaten the postoperative outcomes and survival rate (6-8). The malnutrition often gets worse in early postoperative period which considerably affects mortality, wound healing, intestinal barrier function and postoperative complication rate (9,10). Therefore, researchers have sought for decades to prove whether clinical outcomes can be improved by the administration of postoperative nutritional supports, among which total parenteral nutrition (TPN) and early enteral nutrition (EEN) are the most common ones (11).
It has been debated which nutrition way is better for patients after PD, but the results never reached consistent. A recent randomized multicenter controlled trial compared the outcomes between EEN and TPN after PD, in terms of postoperative complications (12). It concluded that EEN group was associated with a significantly increased overall postoperative complications rate, such as pancreatic fistula. However, a meta-analysis performed by Shen showed early EEN appeared safe and tolerated for patients after PD, but did not show advantages in postoperative infection and hospital stay than TPN (13).
Although some studies have compared the clinical outcomes between EEN and TPN after PD, the data on patients with pancreatic cancer are still limited and conclusions remain unclear. In the present analysis, we aim to compare the short-term clinical outcomes of early EEN versus TPN in patients undergoing PD due to pancreatic cancer.
Methods
Search strategy and selection criteria
The protocol of the meta-analysis has been approved and registered in PROSPERO database (University of York, York, UK) with a registration number of CRD42019121932. Two independent reviewers performed a comprehensive and systematic literature search to identify relevant studies from the databases of PubMed, Embase, Web of Science, Chinese National Knowledge Infrastructure (CNKI) and Wanfang databases. Following keywords with different combinations were used for bibliographic search: “enteral nutrition”, “parenteral nutrition”, “nutrition”, “pancreatic cancer”, “pancreaticoduodenectomy” and “Whipple”. The searches were limited to human studies and both English- and Chinese-language literature. The last search date was December 31, 2018.
Inclusion criteria were: (I) randomized controlled trials (RCTs) reporting the short-term (seven to ten days after surgery) clinical outcomes between EEN (including oral intake, jejunostomy and nasojejunum) and TPN; (II) at least 15 participants in each group; (III) patients pathologically diagnosed with pancreatic ductal adenocarcinoma (PDAC) and underwent PD; (IV) nutritional supports initiating on the first postoperative day and lasted more than ten days; (V) patients treated with isonitrogenous and isocaloric nutrients; (VI) studies available to get complete data. And exclusion criteria were: (I) duplicated papers failing to provide supplementary information; (II) unfinished studies or unavailable data. Literatures were selected by two researchers, and any disagreements were resolved by consensus or judged by the senior authors.
Data extraction and quality assessment
Full-text reviews and quality assessment were conducted for articles which passed the primary selection by two reviewers independently. CONSORT statement (14) was used to measure the quality of randomized controlled trials (RCTs). Reviewers evaluated the risk of bias according to the Cochrane Handbook for Systematic Reviews of Interventions. Any disagreements were resolved through consensus.
Extracted information included: (I) characteristics of studies and patients; (II) basic management of nutrition support; (III) short-term (seven to ten days after surgery) nutritional status, bowel function, mortality and complication events (infection, pancreatic fistula, delayed gastric emptying (DGE), postoperative hemorrhage, total complications); (IV) time of hospital stay.
Statistical analysis
We performed the meta-analysis using RevMan 5.3 software (Cochrane Collaboration, Copenhagen, Denmark). Weighted mean difference (WMD) with 95% confidence interval (CI) was calculated for continuous variables and relative risk (RR) with 95% CI was calculated for dichotomous variables. Forest plots were presented graphically for short-term nutritional status and bowel function. Statistical heterogeneity among studies was calculated using chi-squared test and the I-squared measure on a scale of 0–100% (less than 50% represented a low heterogeneity, 50–75% indicated a moderate inconsistency and higher than 75% meant a large degree of heterogeneity). Fix-effect model was conducted in analysis with heterogeneity <50% and random-effect model was used with heterogeneity ≥50%. Publication bias of total complications was assessed using funnel plot. Two-sided P<0.05 was considered to be statistically significant.
Results
Literature selection and characteristics of studies
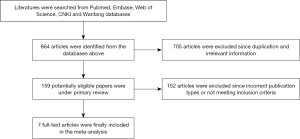
The process of literature selection for eligible studies and exclusion reasons was presented as a flow diagram in Figure 1. Initially, 864 published articles were identified (104 from PubMed, 250 from Embase, 146 from Web of Science, 83 from CNKI and 281 from Wanfang database). Overall, 159 unduplicated articles were selected from these citations. Finally, seven RCTs with a total of 486 patients were included in the present analysis (15-21). The basic characteristics of included studies were presented in Table 1. Among the included patients, 245 (50.4%) subjects got EEN while 241 (49.7%) patients received TPN. Patients in EEN group received nutritional supplementation by placing a nutrition tube in the jejunum within 24 h postoperatively. Subjects in both groups were treated with isonitrogenous and isocaloric nutrients per day.
Table1
| Author (Year) | Country | Sample size | Operation procedure | Administration | EEN | TPN | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | Age (year) | BMI (kg/m2) | TP (g/L) | ALB (g/L) | Number | Age (year) | BMI (kg/m2) | TP (g/L) | ALB (g/L) | ||||||
| Di Carlo, 1999 (15) | Italy | 67 | PD or PPPD | Jejunostomy | 35 | 61.7±12.0 | – | – | 38.0±4.4 | 32 | 62.4±11.3 | – | – | 37.4±4.2 | |
| Wen, 2007 (16) | China | 60 | PD | Jejunostomy | 30 | 67.6±11.5 | 26.04±2.3 | 64.3±9.2 | 41.7±3.6 | 30 | 67.3±15.5 | 26.4±2.6 | 63.3±3.3 | 38.9±4.6 | |
| Liu, 2011 (17) | China | 58 | PD | Jejunostomy | 28 | 59.7±11.2 | 22.5±1.1 | 64.2±6.2 | – | 30 | 60.5±11.9 | 22.9±0.8 | 64.9±5.8 | – | |
| Zhan, 2012 (18) | China | 40 | PD | nasojejunum | 20 | 62.9 | 20.37±2.1 | 40.1±4.1 | – | 20 | 62.9 | 20.1±2.0 | – | 39.9±4.0 | |
| Park, 2012 (19) | Korea | 38 | PD or PPPD | nasojejunum | 18 | 62.7±10.3 | 23.8±3.9 | 69.0±6.0 | 38.0±5.0 | 20 | 61.3±13.2 | 23.5±2.1 | 71.0±5.0 | 40.0±4.0 | |
| Guo, 2013 (20) | China | 178 | PD | Jejunostomy | 90 | 69.0±11.0 | – | – | 35±5 | 88 | 68.0±10.0 | – | – | 36.0±6.0 | |
| Yao, 2016 (21) | China | 45 | PD | nasojejunum | 24 | 51.1±8.2 | 21.6±3.8 | – | 34.3±6.7 | 21 | 48.8±9.6 | 23.1±4.2 | – | 33.8±5.3 | |
EEN, early enteral nutrition; TPN, total parenteral nutrition; BMI, body mass index; TP, total protein; ALB, albumin; PD, pancreaticoduodenectomy; PPPD, pylorus-preserving pancreaticoduodenectomy.
Nutritional status
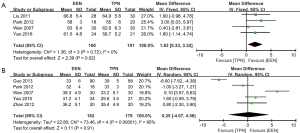
At baseline before surgery, EEN group and TPN group had similar level of plasma total protein (TP) (WMD: –2.24, 95% CI: –6.86–2.38, P=0.34; P for heterogeneity =0.01, I2=77%) and albumin (ALB) (WMD: 0.14, 95% CI: –1.25–1.54, P=0.84; P for heterogeneity = 0.04, I2 = 56%). As shown in Figure 2, after surgery, patients in EEN group had significantly higher plasma TP than those in TPN group (WMD: 1.83, 95% CI: 0.33–3.32, P=0.02; P for heterogeneity =0.72, I2=0%) (Figure 2A), while the ALB level was similar between the groups (WMD: 0.25, 95% CI: –4.07–4.56, P=0.91; P for heterogeneity <0.00001, I2=95%) (Figure 2B).
Bowel function
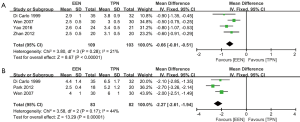
As illustrated in Figure 3, patients in EEN group had shorter exhaust time (WMD: –0.66, 95% CI: –0.81 to –0.51, P<0.00001; P for heterogeneity =0.28, I2 =21%) (Figure 3A) and bowel movement time (WMD: –2.27, 95% CI: –2.61 to –1.94, P<0.00001; P for heterogeneity =0.17, I2 =44%) (Figure 3B) than those in TPN group after surgery.
Complication rate and hospital stay
EEN group had lower rate of short-term total complication (RR: 0.68, 95% CI: 0.51–0.92, P=0.01; P for heterogeneity =0.19, I2=33%) and postoperative hemorrhage rate (RR: 0.22, 95% CI: 0.06–0.75, P=0.02; P for heterogeneity =0.82, I2=0%) than TPN group, while there was no significant difference in infection rate (RR: 0.68, 95% CI: 0.38–1.22, P=0.20; P for heterogeneity =0.87, I2=0%), pancreatic fistula rate (RR: 0.63, 95% CI: 0.35–1.16, P=0.14; P for heterogeneity =0.45, I2 =0%) and DGE rate (RR: 0.72, 95% CI: 0.39–1.33, P=0.29; P for heterogeneity =0.27, I2=23%) between the groups. Only Di Carlo et al. reported a mortality rate of 6.2% in TPN group due to cardiac failure or respiratory failure (15). In addition, EEN group had shorter hospital stay (WMD: –1.53, 95% CI: –2.12 to –0.94, P<0.001; P for heterogeneity =0.49, I2=0%) (Table 2).
Table 2
| Outcomes | Number of studies | Total numbers of patients | RR or WMD | 95% CI | P value |
|---|---|---|---|---|---|
| Total complications | 6 | 426 | 0.68 | [0.51, 0.92] | 0.01 |
| Infection | 6 | 426 | 0.68 | [0.38, 1.22] | 0.20 |
| Pancreatic fistula | 6 | 426 | 0.63 | [0.35, 1.16] | 0.14 |
| DGE | 6 | 426 | 0.72 | [0.39, 1.33] | 0.29 |
| Postoperative hemorrhage | 6 | 426 | 0.22 | [0.06, 0.75] | 0.02 |
| Hospital stay | 6 | 446 | –1.53 | [–2.12, –0.94] | <0.001 |
EEN, early enteral nutrition; TPN, total parenteral nutrition; RR, relative risk; WMD, weighted mean difference; CI, confidence interval; DGE, delayed gastric emptying.
Heterogeneity
In the current analysis, no obvious heterogeneity was found among studies in plasma TP, bowel function, complication events and hospital stay (P for heterogeneity >0.05, I2<50%), except for plasma ALB, with a relatively high heterogeneity (P for heterogeneity <0.001, I2=95%). The discrepancy between Wen and Luo (16) and Guo et al. (20) accounts for the main heterogeneity in ALB.
Publication bias
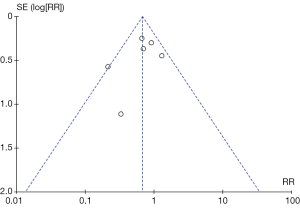
Funnel plot of short-term total complication rate was established in Figure 4, and there was no evident publication bias among studies by visual examination.
Discussion
Nowadays, surgical resection becomes a standard of care to patients with pancreatic cancer without either metastatic or locally advanced disease (22). In the present study, EEN showed better outcomes in improving postoperative plasma TP in patients with pancreatic cancer. As we know, those patients are more vulnerable to get postoperative malnutrition, with a sign of hypoproteinemia or hypoalbuminemia, due to surgical trauma and long operation time (23). And malnutrition is now considered as a risk factor for impaired systemic and intestinal function, as well as decreased digestive and absorptive capacity after surgery (24). TPN provides nutritional support via central venous catheterization when patients are incapable of absorbing nutrients by the gastrointestinal tract. It can benefit postoperative outcomes in the severely malnourished patients and rapidly improve nitrogen balance and wound healing (25). However, catheter complications and overfeeding were main disadvantages of TPN, which might lead to various infections, hyperglycemia or hyperglycemia, metabolic disorders and even death (26). Therefore, EEN became increasingly recognized and was sometimes regarded as the first-line therapy (27).
We also detected EEN helped to improve bowel function by reducing exhaust time and bowel movement time. Pancreatic cancer patients appear to suffer from a set of micronutrient deficiencies and altered bowel function after PD (28). It has been reasonably inferred that EEN protected bowel function by maintaining gastrointestinal integrity thus preventing villous atrophy, and attenuating the body’s response to stress (29). In vivo studies proved that TPN led to impairment of gut mucosal immunity in mice, while partial EEN was able to reverse TPN-induced bowel function impairment through activation of the JAK1-STAT6 pathway (30). Recently, some researchers also demonstrated that early EEN attenuated experimental colitis by inhibiting p65 activation via regulating the p38/MSK1 pathway, suggesting EEN was effective in the treatment of inflammatory bowel disease (31). However, the underlying mechanism of action on EEN and intestinal barrier function remains still unclear and needs further investigations.
Another important finding in our study was the efficacy of EEN to reduce postoperative complication rate, especially the postoperative hemorrhage. Although the techniques in surgical process have been well developed, postoperative complications still threaten the survivor rate and life quality of those patients. Because of surgical complications and patients’ poor status, it has been reported that 15–30% of the postoperative patients may not be candidates for any adjuvant therapy (32). Verma reviewed 30 relative articles and stated the most common postoperative complications after PD for pancreatic cancer included DGE (17–24%), infections (17–20%), pancreatic fistula (10–20%), anastomotic leaks (0–15%) and postoperative hemorrhage (2–13%) (33). Patients with major complications (including pancreatic fistula, DGE and hemorrhage) had significantly worse recurrence-free survival (RFS) and overall survival (OS) than those without major complications after PD for pancreatic cancer (34,35). Researchers have also compared the clinical outcomes between EEN and TPN in patients receiving radical gastrectomy and esophagectomy for gastric cancer and esophageal cancer, respectively. EEN showed better outcomes in terms of maintaining better nutritional status, fewer postoperative complications and shorter hospitalization time (36,37). However, some investigations drew the different conclusions. A review performed by Buscemi stated EEN did not reveal any advantages in terms of pancreatic fistula, post-pancreatectomy hemorrhage, hospital stay and infectious complications (38). Lu and colleagues retrospectively analyzed outcomes between TPN and EEN + PN in patients after PD. And they reported that the EEN + PN group had an increased morbidity of DGE and pulmonary infection, which led to longer nasogastric tube retention time and postoperative hospital stay (39). Therefore, EEN might be performed scrupulously and selectively.
Besides EEN and TPN, other nutrition methods have been developed and applied to reduce the complication rate of pancreatic surgery and to improve life quality in pancreatic cancer patients. A prospective single-center study compared the outcomes between EEN and combined nutrition support (EEN + PN) in patients after PD (40). The rate of discontinuance of enteral feeding was significantly higher in the EEN group, and there was no statistical difference in the frequency of catheter-related infections between the two groups. A recent RCT study tried to evaluate the effectiveness of enteral immunonutrition (EIN) by adding mediators of systemic immunity (interleukin-1α, tumor necrosis factor-α, lymphocytes subsets and complement components) (41). The perioperative administration of EIN rather than EEN was associated with a favorable modulation of inflammatory response and with enhancement of systemic immunity in patients undergoing PD for periampullary cancer. In addition, some investigations also recommended adding omega-3 fatty acids and L-carnitine supplement to prevent severe cachexia and to improve nutritional status (42). Compared to standard EEN and TPN, an enteral formula enriched with omega-3 fatty acids showed better results in term of lower rate of postoperative complications (43). Moreover, early oral feeding after bowel movement has been reported as a clinically safe, feasible and effective method of nutritional support after PD (44). Therefore, patients undergoing pancreatic cancer surgery should receive multidisciplinary nutrition screening and intervention, and the nutrition professionals should be also included in managing these patients in the postoperative period (45).
Some limitations of the present study should be also emphasized. Firstly, this meta-analysis included only 486 patients and 7 RCTs. Some included studies contain relatively small samples (fewer than 20 patients) and remain imbalance of patient number between groups so that deviations may inevitably exist. Secondly, due to the limited data provided by the included studies, we cannot analyze and compare the preoperative data between the groups. Thirdly, some important indices such as prealbumin, transferrin, hemoglobin and immune function are not analyzed due to unavailable data. Fourthly, we cannot perform subgroup analysis according to patient’s gender, age and tumor stage for the lack of relevant information. In addition, the included studies did not mention the enhanced recovery program, which should be also regarded as an important postoperative management. Finally, some uncontrolled factors may interfere with the current analysis. Variables like gender ratio and age range at baseline have not been adjusted. And different managements of surgical process [PD and pylorus-preserving pancreaticoduodenectomy (PPPD)] nasojejunal tube (types, places), and supplemental nutrition may also disturb the accuracy of results.
Conclusions
EEN was better than TPN at improving the nutritional status and bowel function as well as to decreasing complication rate and hospital stay after PD in patients with pancreatic cancer. Novel nutrition support should be also investigated and developed as an adjunctive therapy to pancreatic cancer patients.
Acknowledgments
The authors thank all participants for their participation.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.07.47). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- International Agency for Research on Cancer, World Health Organization. Global Cancer Observatory. 2018. Available online: http://gco.iarc.fr
- Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol 2016;22:9694-705. [Crossref] [PubMed]
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Hidalgo M, Cascinu S, Kleeff J, et al. Addressing the challenges of pancreatic cancer: future directions for improving outcomes. Pancreatology 2015;15:8-18. [Crossref] [PubMed]
- Vincent A, Herman J, Schulick R, et al. Pancreatic cancer. Lancet 2011;378:607-20. [Crossref] [PubMed]
- Chan C, Franssen B, Domínguez I, et al. Impact on quality of life after pancreatoduodenectomy: a prospective study comparing preoperative and postoperative scores. J Gastrointest Surg 2012;16:1341-6. [Crossref] [PubMed]
- Bradley EL 3rd. Long-term survival after pancreatoduodenectomy for ductal adenocarcinoma: the emperor has no clothes? Pancreas 2008;37:349-51. [Crossref] [PubMed]
- La Torre M, Ziparo V, Nigri G, et al. Malnutrition and pancreatic surgery: prevalence and outcomes. J Surg Oncol 2013;107:702-8. [Crossref] [PubMed]
- Schnelldorfer T, Adams DB. The effect of malnutrition on morbidity after Surgery for chronic pancreatitis. Am Surg 2005;71:466-72; discussion 472-3. [PubMed]
- Grizas S, Gulbinas A, Barauskas G, et al. A comparison of the effectiveness of the early enteral and natural nutrition after pancreatoduodenectomy. Medicina (Kaunas) 2008;44:678-86. [Crossref] [PubMed]
- Perinel J, Mariette C, Dousset B, et al. Early Enteral Versus Total Parenteral Nutrition in Patients Undergoing Pancreaticoduodenectomy: A Randomized Multicenter Controlled Trial (Nutri-DPC). Ann Surg 2016;264:731-7. [Crossref] [PubMed]
- Shen Y, Jin W. Early enteral nutrition after pancreatoduodenectomy: a meta-analysis of randomized controlled trials. Langenbecks Arch Surg 2013;398:817-23. [Crossref] [PubMed]
- Schulz KF, Altman DG, Moher D, et al. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [Crossref] [PubMed]
- Di Carlo V, Gianotti L, Balzano G, et al. Complications of pancreatic surgery and the role of perioperative nutrition. Dig Surg 1999;16:320-6. [Crossref] [PubMed]
- Wen B, Lou LG. A clinical study of perioperative nutritional support in patients with pancreaticoduodenectomy. Parenter Enter Nutr 2007;14:219-22,225.
- Liu C, Du Z, Lou C, et al. Enteral nutrition is superior to total parenteral nutrition for pancreatic cancer patients who underwent pancreaticoduodenectomy. Asia Pac J Clin Nutr 2011;20:154-160. [PubMed]
- Zhan C, Xu H, Zhao L, et al. The clinical research of enteral and parenteral nutritional support in postoperative patients of pancreaticoduodenectomy. Pract Onco J 2012;26:225-7,258.
- Park JS, Chung HK, Hwang HK, et al. Postoperative nutritional effects of early enteral feeding compared with total parental nutrition in pancreaticoduodectomy patients: a prosepective, randomized study. J Korean Med Sci 2012;27:261-7. [Crossref] [PubMed]
- Guo JC, Li J, Hu Y, et al. The role of perioperative enteral and parenteral nutrition treatment in pancreatic cancer: a multicenter, prospective randomized controlled trial Chin J Surg 2013;51:987-90. (in Chinese). [PubMed]
- Yao YT, Xiang GM, Xue H, et al. A clinical study on the sequential enteral nutritional therapy after pancreaticoduodenectomy. Pract J Clin Med 2016;13:106-8.
- Giuliano K, Ejaz A, He J. Technical aspects of pancreaticoduodenectomy and their outcomes. Chin Clin Oncol 2017;6:64. [Crossref] [PubMed]
- Gilliland TM, Villafane-Ferriol N, Shah KP, et al. Nutritional and Metabolic Derangements in Pancreatic Cancer and Pancreatic Resection. Nutrients 2017;9:E243. [Crossref] [PubMed]
- Abunnaja S, Cuviello A, Sanchez JA. Enteral and parenteral nutrition in the perioperative period: state of the art. Nutrients 2013;5:608-23. [Crossref] [PubMed]
- Bozzetti F. Peri-operative nutritional management. Proc Nutr Soc 2011;70:305-10. [Crossref] [PubMed]
- Worsh CE, Tatarian T, Singh A, et al. Total parenteral nutrition in patients following pancreaticoduodenectomy: lessons from 1184 patients. J Surg Res 2017;218:156-61. [Crossref] [PubMed]
- Klek S, Sierzega M, Szybinski P, et al. Perioperative nutrition in malnourished surgical cancer patients-a prospective, randomized, controlled clinical trial. Clin Nutr 2011;30:708-13. [Crossref] [PubMed]
- Armstrong T, Walters E, Varshney S, et al. Deficiencies of micronutrients, altered bowel function, and quality of life during late follow-up after pancreaticoduodenectomy for malignancy. Pancreatology 2002;2:528-34. [Crossref] [PubMed]
- Fujita T, Daiko H, Nishimura M. Early enteral nutrition reduces the rate of life-threatening complications after thoracic esophagectomy in patients with esophageal cancer. Eur Surg Res 2012;48:79-84. [Crossref] [PubMed]
- Sun H, Bi J, Lei Q, et al. Partial enteral nutrition increases intestinal sIgA levels in mice undergoing parenteral nutrition in a dose-dependent manner. Int J Surg 2018;49:74-9. [Crossref] [PubMed]
- Yu T, Yu Q, Chen X, et al. Exclusive enteral nutrition protects against inflammatory bowel disease by inhibiting NF-κB activation through regulation of the p38/MSK1 pathway. Int J Mol Med 2018;42:130516.
- Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg 2000;4:567-79. [Crossref] [PubMed]
- Verma V, Li J, Lin C. Neoadjuvant Therapy for Pancreatic Cancer: Systematic Review of Postoperative Morbidity, Mortality, and Complications. Am J Clin Oncol 2016;39:302-13. [Crossref] [PubMed]
- Watanabe Y, Nishihara K, Matsumoto S, et al. Effect of postoperative major complications on prognosis after pancreatectomy for pancreatic cancer: a retrospective review. Surg Today 2017;47:555-67. [Crossref] [PubMed]
- Futagawa Y, Kanehira M, Furukawa K, et al. Impact of delayed gastric emptying after pancreaticoduodenectomy on survival. J Hepatobiliary Pancreat Sci 2017;24:466-74. [Crossref] [PubMed]
- Wang J, Zhao J, Zhang Y, et al. Early enteral nutrition and total parenteral nutrition on the nutritional status and blood glucose in patients with gastric cancer complicated with diabetes mellitus after radical gastrectomy. Exp Ther Med 2018;16:321-7. [PubMed]
- Peng J, Cai J, Niu ZX, et al. Early enteral nutrition compared with parenteral nutrition for esophageal cancer patients after esophagectomy: a meta-analysis. Dis Esophagus 2016;29:333-41. [Crossref] [PubMed]
- Buscemi S, Damiano G, Palumbo VD, et al. Enteral nutrition in pancreaticoduodenectomy: a literature review. Nutrients 2015;7:3154-65. [Crossref] [PubMed]
- Lu JW, Liu C, Du ZQ, et al. Early enteral nutrition vs parenteral nutrition following pancreaticoduodenectomy: Experience from a single center. World J Gastroenterol 2016;22:3821-8. [Crossref] [PubMed]
- Nagata S, Fukuzawa K, Iwashita Y, et al. Comparison of enteral nutrition with combined enteral and parenteral nutrition in post-pancreaticoduodenectomy patients: a pilot study. Nutr J 2009;8:24. [Crossref] [PubMed]
- Hamza N, Darwish A, O'Reilly DA, et al. Perioperative Enteral Immunonutrition Modulates Systemic and Mucosal Immunity and the Inflammatory Response in Patients With Periampullary Cancer Scheduled for Pancreaticoduodenectomy: A Randomized Clinical Trial. Pancreas 2015;44:41-52. [Crossref] [PubMed]
- Gärtner S, Krüger J, Aghdassi AA, et al. Nutrition in Pancreatic Cancer: A Review. Gastrointest Tumors 2016;2:195-202. [Crossref] [PubMed]
- Gianotti L, Braga M, Gentilini O, et al. Artificial nutrition after pancreaticoduodenectomy. Pancreas 2000;21:344-51. [Crossref] [PubMed]
- Hwang SE, Jung MJ, Cho BH, et al. Clinical feasibility and nutritional effects of early oral feeding after pancreaticoduodenectomy. Korean J Hepatobiliary Pancreat Surg 2014;18:84-9. [Crossref] [PubMed]
- Afaneh C, Gerszberg D, Slattery E, et al. Pancreatic cancer surgery and nutrition management: a review of the current literature. Hepatobiliary Surg Nutr 2015;4:59-71. [PubMed]