
Alpha-actinin 4 and tumorigenesis of hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is the most common primary malignant liver tumor, and its mortality rate ranks third among those of all tumors worldwide (1). Although many studies have demonstrated that oncogene amplification or deletions of tumor suppressor genes may cause cancers (2-4), the specific cellular and molecular mechanisms of tumorigenesis are still not fully understood. In recent years, with technological innovations in the diagnosis of and treatment methods for HCC, the current survival rate of patients with HCC has been greatly extended; however, tumor metastasis and recurrence are still the main factors that affect the survival of patients (5,6). Therefore, a thorough understanding of the underlying molecular mechanisms associated with the occurrence and development of HCC that focuses on the identification of the molecular targets related to the prognosis of HCC and on the exploration of effective interventions will help to clarify the pathogenesis of HCC and aid in the development of new treatment strategies.
It is reported that over 75% of all HCC cases are due to chronic infection with hepatitis B virus (HBV) or hepatitis C virus (HCV) (5,6). HBV, a DNA virus, and HCV, an RNA virus, both increase the risk of HCC in those infected. HBV contains a partially double stranded genomic DNA that does not encode a dominant oncogene. Thus, HBV is thought to be carcinogenic in an indirect fashion, such as by insertional activation of cellular oncogenes the induction of genetic instability upon HBV DNA integration or by the regulatory protein HBx. HCV is a small enveloped positive-sense, single stranded RNA virus. There is no DNA intermediate in the replication of the HCV genome or integration of viral nucleic acid and viral pathology may contribute to oncogenesis through cirrhosis and regeneration of liver cells. When HCV replicates in hepatocytes, it causes changes in liver cell structure and function or interferes with hepatocyte protein synthesis, which can cause degeneration and necrosis of hepatocytes. The molecular mechanisms underlying hepatocarcinogenesis remain far from understood, including the responsible etiological factors, the marked genetic and epigenetic heterogeneity, as well as the cell types at the origin of transformation. There are many studies focus on the molecular mechanisms underlying hepatitis virus-induced hepatocarcinogenesis, as well as oncogenic pathways that are activated particularly at late stages of the transformation process in virus and non-virus induced hepatocarcinogenesis (6). Viral eradication is the common effective strategy to reduce the risk of HCC development from HBV and HCV patients (7). However, for those who have already developed cirrhosis, it does not work. Cirrhosis is an advanced form of liver disease that has many causes (8). A damaged liver caused by alcoholism or chronic infections like hepatitis B and C can lead to cirrhosis. It is the biggest risk factor for liver cancer, and is considered as a preneoplastic state (8). Cirrhosis usually occurs when the liver is damaged over an extended period of time. When liver cells are damaged and die due to chronic disease of the liver, fibrosis is activated. Fibrous scar tissue can be deposited in place of the missing cells. The scar tissue blocks the flow of blood through the liver and slows the liver’s ability to process nutrients, hormones, drugs, and natural toxins. This impairs the hepatic functions. HCC is one serious complication that develops as a result of chronic cirrhosis (9).
The key difference between liver cirrhosis and HCC is that liver cancer can spread to the adjacent organs and then into the distant sites because of the invasive nature of the malignant cells whereas cirrhosis is confined to the liver (10). In the late stage of cancer, metastasis can occur when cancer cells break away from the primary tumor, where the cancer began. There is an increase of evidence supporting that alpha-actinin 4 (ACTN4) is associated with cancer cell motility (11). ACTN4 is a member of the spectrin superfamily, widely expressed, and cross-links with actin microfilaments to maintain cytoskeletal integrity and control cell motility (12).
ACTN4 has been demonstrated to be involved in cytoskeletal reorganization and the regulation of cell adhesion and morphology. Silencing ACTN4 expression significantly reduces the number of oral squamous cells (13), glioma cells (14), and astrocytoma cells (15) and a variety of tumor movements and invasive abilities. However, no research has focused on the role of ACTN4 in the development of HCC or its clinical relevance to HCC. In this study, the expression of ACTN4 in HCC tissues was measured by quantitative reverse transcription polymerase chain reaction (qRT-PCR) and immunohistochemistry. The relationships between the expression of ACTN4 in HCC and the clinicopathological features and postoperative survival rates of HCC patients were analyzed. We studied the expression of ACTN4 in hepatocytes and its prognostic value.
Methods
Materials
A total of 87 HCC specimens were collected from the Department of Hepatobiliary Surgery of Linyi Hospital of Traditional Chinese Medicine from December 2005 to December 2010 and were confirmed by pathology and follow-up data. The clinical-pathological demographic information is available in Table 1. All patients without any anti-tumor therapies before surgery were included. The tumor tissues and corresponding paracancerous tissues were collected according to the experimental design of the tissue chips. The postoperative follow-ups were conducted at 3 months with telephone interviews. The follow-up time was 3 months after the patient was discharged from the hospital, and the deadline was the end of the study or December 31, 2015. Lost cases were not included in the final statistical analyses of this study. The study was approved by the Ethics Committee of Linyi Hospital of Traditional Chinese Medicine, and all patients enrolled in the study agreed to the use of their tissue samples and provided written informed consent.
Table 1
| Clinicopathological features | n | ACTN4 expression | P value | |
|---|---|---|---|---|
| Positive (n=47) | Negative (n=40) | |||
| Age | 0.894 | |||
| ≤50 years | 42 | 23 | 19 | |
| >50 years | 45 | 24 | 21 | |
| Gender | 0.989 | |||
| Male | 74 | 40 | 34 | |
| Female | 13 | 7 | 6 | |
| Tumor size | 0.498 | |||
| ≤5 cm | 36 | 21 | 15 | |
| >5 cm | 51 | 26 | 25 | |
| Tumor number | 0.405 | |||
| Single | 66 | 34 | 32 | |
| Multiple | 21 | 13 | 8 | |
| Lymph node status | 0.046 | |||
| Present | 8 | 7 | 1 | |
| Absent | 79 | 40 | 39 | |
| Edmonson-Steiner grade | 0.237 | |||
| 1, 2 | 64 | 37 | 27 | |
| 3, 4 | 23 | 10 | 13 | |
Experimental reagents and instruments
Rabbit anti-human ACTN4 polyclonal antibody was purchased from Abcam. The concentrated DAB Chromogenic Kit and ready-to-use immunohistochemical Elivision Super Test kit were purchased from Fuzhou Maixin Science and Technology Co., Ltd. The TRIzol cell lysates were purchased from Life Technologies (Thermo Fisher Scientific Inc.). The RNA reverse transcription reagent was purchased from the TaKaRa company.
Methods
Immunohistochemistry
Conventional xylene dewaxing and gradient alcohol dehydration were applied. The samples were repaired with a high-pressure treatment comprising citrate buffer for 90 s, incubated in 30 mL/L hydrogen peroxide for 25 min and rinsed 5 times in phosphate buffer for 5 min/wash. The samples were then incubated with rabbit anti-human ACTN4 primary antibody (1:150) overnight at 4 °C and rinsed 5 times in phosphate buffer for 5 min/wash. First, a polymer enhancer was added, and the tissues were then incubated at 37 °C for 15 min. Later, the samples were washed in phosphate buffer 5 times for 5 min/wash. Second, the enzyme-labeled anti-rabbit polymer amplification agent was added, the samples were incubated at 37 °C for 15 min, and a phosphate buffer wash was applied 5 times for 5 min/wash. The DAB color and hematoxylin counterstain were added, and 1 mL/L hydrochloric acid was applied before alcohol differentiation. When the PBS turned blue, the samples were dehydrated and sealed when they were transparent. The results determined the reference judgments (16). On each film, 10 fields were selected, 100 cells were counted in each field, and the percentage of positive cells and the degree of staining were determined. The immunohistochemical results were evaluated by two pathologists under a microscope in a double-blind manner. Inconsistencies between the results were resolved by discussion to obtain consistent results. Indeed, the intensities of staining were indicated as follows: 0, no staining; 1 light staining (or less than 25% positive cells); 2 moderate staining (25% to 50% positive cells); and 3 for strong staining (>50% positive cells). Staining was also dichotomized into negative and positive. Negative was scored for 0 and 1 and positive was scored if 2 and 3 (17,18).
RNA extraction and qRT-PCR
Total RNA was extracted from transfected cells using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer’s instructions. The first-strand cDNA was generated using a High-Capacity cDNA Reverse Transcription kit (TAKARA BIO INC, Otsu, Japan). Gene expression levels were determined by qRT-PCR using a real-time PCR system (Bio-Rad) with the following primers: ACTN4 (5'-ACAAGCCCAACCTGGAC-3' and 5'-GGTGCGGGCAATGGTG-3') (19). The β-actin was used as an endogenous control for RT-PCR amplification measurements of ACTN4 expression. RT-PCR primers for β-actin: 5'-CTGGAACGGTGAAGGTGACA-3' and 5'-AAGGGACTTCCTGTAACAATGCA-3' (19). The qRT-PCR was conducted based on following conditions: pre-denaturation at 95 °C for 3 min, followed by 40 cycles of denaturation at 95 °C for 12 s, annealing and extension at 60 °C for 35 s.
Statistical analysis
The experimental data were statistically analyzed with IBM SPSS 22.0 and are expressed as the means ± SD. The data were compared between groups with t-tests. The differences in ACTN4 expression between the cancer tissues and adjacent tissues were examined with chi-square tests, and the relationships between ACTN4 expression and the clinicopathological features of the patients with HCC were examined using the Mann-Whitney U test. A Kaplan-Meier survival curve was calculated, and the difference in survival between the groups was examined using the log-rank test. We have also performed one-way Multivariate Analysis of Variance (MANOVA) by using Matlab software. It performed with MANOVA function for comparing the multivariate means of the grouped data, and determine whether the mean of a variable differs significantly among groups. P<0.05 was taken to indicate a significant difference.
Results
ACTN4 is highly expressed in HCC
The qRT-PCR results indicated that the expression of ACTN4 in the HCC tissues were significantly higher than that in the paracancerous tissues (Figure 1, P<0.05). All the patients were divided into two groups based on the PCR results of ACTN4 expression. The patients who had higher ACTN4 expression in the HCC tissues than in the paracancerous tissues, as detected by the PCR analysis, were labeled as ACTN4-positive; otherwise, they were labeled as ACTN4-negative. The immunohistochemistry results indicated that the expression of ACTN4 was upregulated in the HCC tissues as illustrated in Figure 2 and that the upregulation rates are significantly higher than those in the adjacent tissues (as illustrated in Figure 3). The ACTN4-positive staining of the cancer cells indicated that the stained granules in the nucleus and cytoplasm were manifested as brown granules. The results of the comprehensive analysis of the 87 cases of HCC in the patients with cancer tissues and adjacent tissues for ACNT4 protein immunohistochemical staining are presented in Table 2. A χ2 test revealed that the staining for ACNT4 differed significantly between the HCC tissues and the adjacent tissues (Table 2, P=0.029).
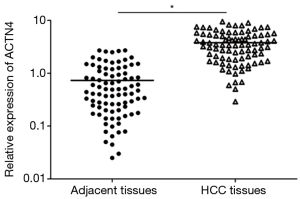
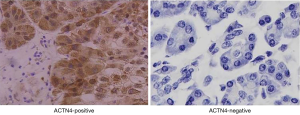
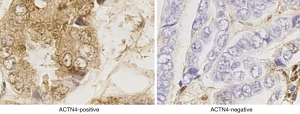
Table 2
| ACTN4 in HCC tissues | ACTN4 in adjacent tissues | Total | |
|---|---|---|---|
| + | − | ||
| + | 6 | 41 | 47 |
| − | 0 | 40 | 40 |
| Total | 6 | 81 | 87 |
The expression levels of ACTN4 in the HCC tissues and adjacent tissues were analyzed with χ2 analysis, with P=0.029. HCC, hepatocellular carcinoma.
ACTN4 and the clinicopathological features of the HCC patients
Mann-Whitney U analysis showed that the upregulation of ACTN4 in HCC was significantly associated with lymph node metastasis (Table 1, P=0.046). The expression of ACTN4 exhibited no significant correlations with clinicopathological features, including gender, age, tumor size, tumor number and Edmondson-Steiner pathological grade.
ACTN4 and the postoperative survival of the HCC patients
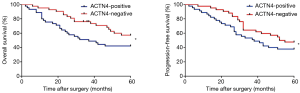
According to the results of the ACTN4 immunohistochemical staining, we divided the patients with HCCs into an ACTN4-positive group and an ACTN4-negative group. In the ACTN4-positive group, the 5-year overall survival rate was 37.044±21.977 months (Figure 4, left panel), and the progression-free survival rate was 33.149±24.147 months (Figure 4, right panel). In the ACTN4-negative group, the 5-year overall survival rate was 50.775±15.272 months (Figure 4, left panel), and the progression-free survival rate was 48.915±18.491 months (Figure 4, right panel). The Kaplan-Meier survival curves revealed that both the 5-year overall survival and progression-free survival rates of the patients with ACTN4-positive HCCs were all significantly lower than those of the ACTN4-negative patients (Manova1 analysis performed in Matlab: P<0.032 overall group, P<0.046 for progression-free group), and the differences were statistically significant.
Discussion
ACTN4 is a fiber-grafted actin cross-linked protein that increases the motility of tumor cells, and it is closely related to tumor metastasis and poor prognosis (20). The underlying mechanism of these processes may be that ACTN4 affects cell motility and tumor metastasis by regulating cytoskeletal organization and adhesion by combining with Rho family molecules (15). In colon cancer, colon cancer cells that overexpress ACTN4 stimulate the activation of aggressive cellular processes and exhibit a more aggressive malignant phenotype than control cells (14). Additionally, the silencing of ACTN4 expression in pancreatic and oral squamous cell carcinomas results in decreased tumor cell invasiveness (21). In colorectal and pancreatic cancers, overexpression of ACTN4 in tumor cells results in lymph node metastases in the tumor area (14,21). More importantly, one study found that the enrichment of ACTN4 in lung cancer is closely related to the poor prognoses of cancer patients (22). Therefore, it is particularly important to study the relationships between ACTN4 expression in HCC and the patients’ clinicopathological features and prognoses.
In this study, we measured the expression levels of ACTN4 by qRT-PCR and immunohistochemical staining in patients with HCCs and examined the relationships between ACTN4 and the clinicopathological features and postoperative survival of patients. The qRT-PCR results revealed that the expression of ACTN4 in the HCCs was significantly higher than that in the paracancerous tissues (P=0.029). Immunohistochemistry indicated that the rate of ACTN4-positive cells in the carcinomas was 54.02 (47/87). The positive rate in the paracancerous tissues was 7.41 (6/81). The difference between the two groups was statistically significant. The above results suggest that ACTN4 may be involved in the development of HCC. Reports in the literature state that ACTN4 is closely related to pancreatic cancer (21), ovarian cancer (23,24), and lung cancer (22) in terms of the occurrence, development and prognoses of these cancers, which suggests that ACTN4 may be located in a common pathway of cancer cells through involvement with the development of tumors.
To further study the relationship between ACNT4 and the development of HCCs, our group analyzed the relationships between the expression of ACTN4 and the clinicopathological characteristics and survival times of HCC patients using statistical methods. Our study found that the positive expression of ACTN4 in HCC cells was significantly correlated with lymph node metastasis, which suggests that ACTN4 may participate in the process of liver cell metastasis and promote tumor progression. More importantly, further statistical analyses revealed that the 5-year overall survival and progression-free survival rates were significantly lower in the ACTN4-positive expression group than in the ACTN4-negative expression group, which suggests that ACTN4 is closely related to HCC recurrence and the long-term survival rate.
Although this study observed that the positive expression of ACTN4 in HCC cells was significantly correlated with lymph node metastasis, which suggests that ACTN4 may participate in the process of liver cell metastasis and promote tumor progression, other factors including microvascular invasion (MVI) or tumor satellite micronodules may play crucial roles for metastases of HCC (25). MVI is a crucial histopathologic prognostic factor for HCC. Early tumor recurrence is linked to increased mortality rate. It was recently reported (26) that MVI is a more prominent tumor recurrence predictor than the Milan criteria for HCC after surgical resection. Imaging evidence suggested that MVI are involved in the disruption of capsule, irregular tumor margin, peritumoral enhancement, multifocal tumor, increased tumor size, and increased glucose metabolism. The correlation between MVI and segmental location of HCC is still an open issue that require further investigation.
Conclusions
Our results suggest that ACTN4 may participate in the progression of HCC and affect the liver cells and postoperative survival rates of cancer patients. This study lays a foundation for the study of the molecular mechanism through which ACTN4 influences the process of HCC metastasis and provides a new target for the prognosis of and targeted therapy for HCC.
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.07.34). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Linyi Hospital of Traditional Chinese Medicine, and all patients enrolled in the study agreed to the use of their tissue samples and provided written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chettouh H, Lequoy M, Fartoux L, et al. Hyperinsulinaemia and insulin signalling in the pathogenesis and the clinical course of hepatocellular carcinoma. Liver International 2015;35:2203-17. [Crossref] [PubMed]
- Porta C, Larghi P, Rimoldi M, et al. Cellular and molecular pathways linking inflammation and cancer. Immunobiology 2009;214:761-77. [Crossref] [PubMed]
- Liu B, Tan X, Liang J, et al. A reduction in reactive oxygen species contributes to dihydromyricetin-induced apoptosis in human hepatocellular carcinoma cells. Sci Rep 2014;4:7041. [Crossref] [PubMed]
- Gu X, Fu M, Ding Y, et al. High expression of MAGE-A9 correlates with unfavorable survival in hepatocellular carcinoma. Sci Rep 2014;4:6625. [Crossref] [PubMed]
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007;132:2557-76. [Crossref] [PubMed]
- Kew MC. Hepatitis B and C viruses and hepatocellular carcinoma. Clin Lab Med 1996;16:395-406. [Crossref] [PubMed]
- Kim DY, Han K. Epidemiology and Surveillance of Hepatocellular Carcinoma. Liver Cancer 2012;1:2-14. [Crossref] [PubMed]
- Fattovich G, Stroffolini T, Zagni I, et al. Hepatocellular carcinoma in cirrhosis: Incidence and risk factors. Gastroenterology 2004;127:S35-S50. [Crossref] [PubMed]
- del Olmo JA, Serra MA, Rodríguez F, et al. Incidence and risk factors for hepatocellular carcinoma in 967 patients with cirrhosis. J Cancer Res Clin Oncol 1998;124:560-4. [Crossref] [PubMed]
- Bolondi L, Gramantieri L. From liver cirrhosis to HCC. Intern Emerg Med 2011;6:93-8. [Crossref] [PubMed]
- Honda K, Yamada T, Endo R, et al. Actinin-4, a novel actin-bundling protein associated with cell motility and cancer invasion. J Cell Biol 1998;140:1383-93. [Crossref] [PubMed]
- Ribeiro Ede A Jr, Pinotsis N, Ghisleni A, et al. The structure and regulation of human muscle alpha-actinin. Cell 2014;159:1447-60. [Crossref] [PubMed]
- Yamada S, Yanamoto S, Yoshida H, et al. RNAi-mediated down-regulation of alpha-actinin-4 decreases invasion potential in oral squamous cell carcinoma. Int J Oral Maxillofac Surg 2010;39:61-7. [Crossref] [PubMed]
- Ehrlicher AJ, Krishnan R, Guo M, et al. Alpha-actinin binding kinetics modulate cellular dynamics and force generation. Proc Natl Acad Sci U S A 2015;112:6619-24. [Crossref] [PubMed]
- Shao H, Shaoyan L, Simon CW, et al. α-Actinin-4 is required for amoeboid-type invasiveness of melanoma cells. J Biol Chem 2014;289:32717-28. [Crossref] [PubMed]
- Jung WB, Kim CW, Yoon YS, et al. Observational Study: Familial Relevance and Oncological Significance of Revised Bethesda Guidelines in Colorectal Patients That Have Undergone Curative Resection. Medicine (Baltimore) 2016;95:e2723. [Crossref] [PubMed]
- Wu D, Wu P, Zhao L, et al. NF-κB Expression and Outcomes in Solid Tumors: A Systematic Review and Meta-Analysis. Medicine 2015;94:e1687. [Crossref] [PubMed]
- Allegra E, Lombardo N, Puzzo L, et al. The usefulness of toluidine staining as a diagnostic tool for precancerous and cancerous oropharyngeal and oral cavity lesions. Acta Otorhinolaryngologica Italica 2009;29:187. [PubMed]
- Gao Y, Li G, Sun L, et al. ACTN4 and the pathways associated with cell motility and adhesion contribute to the process of lung cancer metastasis to the brain. BMC Cancer 2015;15:277. [Crossref] [PubMed]
- Hammer A, Rider L, Oladimeji P, et al. Tyrosyl phosphorylated PAK1 regulates breast cancer cell motility in response to prolactin through filamin A. Mol Endocrinol 2013;27:455-65. [Crossref] [PubMed]
- Shindo K, Aishima S, Ohuchida K, et al. Podoplanin expression in cancer-associated fibroblasts enhances tumor progression of invasive ductal carcinoma of the pancreas. Mol Cancer 2013;12:168. [Crossref] [PubMed]
- Noro R, Honda K, Tsuta K, et al. Distinct outcome of stage I lung adenocarcinoma with ACTN4 cell motility gene amplification. Ann Oncol 2013;24:2594-600. [Crossref] [PubMed]
- Honda K. The biological role of actinin-4 (ACTN4) in malignant phenotypes of cancer. Cell Biosci 2015;5:41. [Crossref] [PubMed]
- Wang MC, Chang YH, Wu CC, et al. Alpha-actinin 4 is associated with cancer cell motility and is a potential biomarker in non-small cell lung cancer. J Thorac Oncol 2015;10:286-301. [Crossref] [PubMed]
- Ünal E, Idilman IS, Akata D, et al. Microvascular invasion in hepatocellular carcinoma. Diagn Interv Radiol 2016;22:125-32. [Crossref] [PubMed]
- Lim KC, Chow PK, Allen JC, et al. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann Surg 2011;254:108-13. [Crossref] [PubMed]


