Plasma cell-free DNA integrity: a potential biomarker to monitor the response of breast cancer to neoadjuvant chemotherapy
Introduction
Breast cancer has become the most frequently diagnosed cancer in women worldwide (1,2). Surgery, systemic therapy, and radiotherapy, as the main treatment modalities, have significantly improved the prognosis of breast cancer (3). In particular, neoadjuvant chemotherapy (NACT), with the advantage of downsizing the tumor before surgery, provides a therapeutic alternative for patients with locally advanced breast cancer (4). However, although some patients that respond to NACT have good prognosis, a considerable proportion of patients gain little or no benefit from such treatment. More seriously, these patients are exposed to the deleterious effects of cytotoxic drugs (5). Therefore, it is particularly important to monitor treatment responses during NACT. Currently, treatment-monitoring methods are limited and the most common evaluation method is radiographic inspection (6). However, radiological imaging cannot properly reflect the state of the disease in real time. Therefore, it is necessary to identify a predictive biomarker to monitor the disease response to NACT, which will be useful for the individualized breast cancer management and the more effective administration of chemotherapeutics (7).
Blood-based biomarkers have some advantages over imaging or histological examination because they can be accessed using minimally invasive procedures, and multiple samples can be obtained over a specific period (8). However, traditional circulating biomarkers, such as carcinoembryonic antigen and carbohydrate antigen 153 (CA153) have low sensitivity and specificity (9). Recently, cell-free DNA (cfDNA) in blood has attracted research attention, and different cancer-associated cfDNA molecular characteristics, including copy number aberrations, methylation changes, single-nucleotide mutations, cancer-derived viral sequences, and chromosomal rearrangements, have been studied extensively (10). Furthermore, cfDNA integrity (cfDI), which measures the extent of cfDNA fragmentation, has also been exploited as a diagnostic and prognostic biomarker in cancer (11). As a biomarker, cfDI has practical advantages. For example, serum can be obtained easily, and the fast and well-established quantitative real-time PCR (qPCR) method can be used, which requires small amounts of blood and is a relatively cost-efficient technology (12). In circulation, DNA has a short half-life, ranging from 15 min to several hours, which can represent the real-time status of a tumor (13,14). Although cfDI could serve as an attractive prognostic marker for patients with metastatic breast cancer at baseline and during systemic therapy (15), results from different studies have been inconsistent. Furthermore, few studies have focused on the clinical significance of cfDI for patients receiving NACT.
In the present study, the cfDNA concentration and cfDI of patients with locally advanced breast cancer were detected during NACT. The aim was to investigate whether the dynamics of the cfDNA concentration and cfDI reflect the therapeutic effect and whether they could be used as biomarkers for real-time monitoring of NACT.
Methods
Subjects and plasma sample preparation
Female patients with locally advanced breast cancer that were clinically diagnosed using tissue biopsy and radiological imaging were included in this study. The basic information of the enrolled patients and their chemotherapy regimens were collected. All patients comprised individuals with no other clinically diagnosed malignancies, autoimmune diseases, or infections. At the time of definitive diagnosis, blood was collected from each patient before they underwent any therapy. The second blood sample was drawn approximately half way through the NACT procedure. All patients were evaluated systematically every two cycles, and we collected peripheral blood during each evaluation period. For patients receiving three to four cycles of NACT, the intermediate time point referred to the assessment period after the second cycle. For patients receiving eight cycles of NACT, the intermediate time point referred to the assessment period after the fourth cycle. The last blood sample was collected before surgery or at the termination of treatment. The histopathological findings at surgery were compared with the diagnostic biopsies.
Peripheral blood (10 mL) was collected and shipped at room temperature within 2 h to the laboratory for immediate processing using Ficoll density gradient centrifugation (STEMCELL Technologies, Vancouver, Canada), according to the manufacturer’s protocol. The supernatant plasma was centrifuged again at 2,000 ×g for 10 min at 4 °C to minimize any contamination from blood cells or cell debris. All samples were stored at −80 °C until further use. This study was approved by the ethical and scientific committee of our institution (Ethics Approval Number: 2010-SR-091. A1, Ethics Committee of the First Affiliated Hospital of Nanjing Medical University) and informed consent was obtained from all participants.
DNA extraction from plasma
DNA was extracted from 200 µL of plasma using a QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol, and the final eluate was collected and stored at −20 °C. Samples were extracted together to avoid batch effects.
Estimation of the cfDNA concentration and cfDI using repetitive elements
The cfDNA concentration and cfDI were derived by analyzing two repetitive elements, Alu and LINE1. These two repetitive elements have a high copy number and are distributed throughout human genomic DNA. They were independently analyzed in parallel for each sample, which ensured the generation of accurate cfDI estimates. For each of the targets, short (Alu =111 bp, LINE1 =97 bp) and long (Alu =260 bp, LINE1 =266 bp) fragments were measured in triplicate using qPCR with an Absolute SYBR Green assay and the Step One Plus Real-Time PCR System (Applied Biosystems, Waltham, MA, USA). Primers were designed according to a previous report (Table S1) (16). PCR was performed using the FastStart Universal SYBR Green Master (Rox, Hofstetten, Germany) according to the manufacturer’s instructions, in a final volume of 20 µL containing 10 µL of 2× SYBR Green, 0.2 µL of 10 µM PCR forward primer, 0.2 µL of 10 µM PCR reverse primer, 1 µL of DNA template, and 8.6 µL of distilled water (dH2O). The thermal cycling conditions were 10 min at 95 °C; followed by 40 cycles of denaturation at 95 °C for 15 s, annealing at 60 °C for 60 s, and extension at 95 °C for 15 s. A known-concentration DNA standard was divided into five copies and diluted to 1, 10, 100, 1,000, and 10,000 ng/mL. DNA concentration-Ct value standard curves were constructed. The respective absolute concentrations of the long and short fragments were calculated and the cfDI was subsequently calculated as the ratio of the long fragment concentration to the short fragment concentration. The total cfDNA concentration of a sample was derived using the short fragment concentration.
Data analysis
Statistical analysis was performed using SPSS 20 (IBM Corporation, Armonk, NY, USA). Comparisons between two groups were made using a paired T test and between three groups was performed using one-way analysis of variance (ANOVA). Correlation coefficients of two variables were calculated using Pearson analysis. Multivariate analysis was performed using multiple regression analysis. In all figures, asterisks denote significance levels as follows: *P<0.05, **P<0.01.
Results
Study inclusion and patient characteristics
Twenty-nine patients with locally advanced breast cancer were included in this study. All subjects were female. Their median age was 44 years old (range: 29 to 67 years old). Twenty-two patients (75.8%) were pre-menopausal. All tumors were diagnosed as invasive ductal carcinoma (IDC). Immunohistochemical analysis showed the marker of proliferation Ki-67 (Ki67) index in four patients was less than 14%. Luminal A [estrogen receptor (ER) and/or progesterone receptor (PR) positive, human epidermal growth factor receptor 2 (HER-2) negative, Ki67 <14%], luminal B (ER and/or PR positive, HER-2 negative, Ki67 high; or ER and/or PR positive, Any Ki67, HER-2 overexpressed or amplified), HER-2 positive (HER-2 over-expressed or amplified, ER and PR absent), and triple negative (ER and PR absent, HER-2 negative) breast cancer accounted for 3.4%, 44.8%, 41.4%, and 10.4%, respectively.
NACT regimens in this study included docetaxel + epirubicin + cyclophosphamide; epirubicin + cyclophosphamide followed by paclitaxel every 2 weeks; epirubicin + cyclophosphamide followed by paclitaxel plus trastuzumab; fluorouracil + epirubicin + cyclophosphamide; and docetaxel + carboplatin + trastuzumab. Table S2 lists the clinical and histological data of the patients.
Standard curve setting
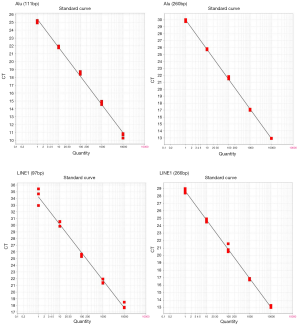
We constructed standard curves for Alu and LINE1. The R2 of each curve was >0.99. The PCR efficiencies of all primer pairs were >70% [Alu(111 bp): 88.92%, Alu(260 bp): 71.55%, LINE1(97 bp): 74.05%, LINE1(266 bp): 80.29%]. Primer specificity was confirmed by melt curve analysis, and no multiple peaks were found. The concentration–Ct value formula of Alu was: Y(111bp) = −3.62X + 25.41, Y(260 bp) = −4.266X + 29.993. The formula of LINE1 was: Y(97 bp) = −4.155X + 34.243,
Y(266 bp) = −3.907X + 28.69 (Figure S1).
DNA concentration and cfDI pre-NACT had no obvious correlation with the patients’ clinical characteristics
Our results showed that the mean concentration of the short Alu fragment was 75.89±71.18 ng/mL (range: 9.23 to 313.50 ng/mL), and the mean cfDI value of Alu was 0.51±0.26 (range: 0.09 to 0.95) before NACT. The mean concentration of the short LINE1 fragment was 82.47±132.93 ng/mL (range: 1.51 to 727.60 ng/mL), and the mean cfDI value of LINE1 was 0.23±0.19 (range: 0.01 to 0.67). We attempted to determine whether the cfDNA concentration or cfDI correlated with the clinical characteristics of the patients. The results showed that the cfDNA concentration or cfDI had no significant correlation with most of the clinical characteristics of the patients, except for the cfDI value of Alu, which correlated positively with the expression of PR (P=0.04). In addition, CA153 levels were investigated, but no positive result was obtained (Table S3).
Breast cancer patients receiving NACT have increasing cfDI values
We sampled peripheral blood from each patient before, in the middle, and at the end of the NACT. CfDNA was extracted from the blood and the cfDNA concentration and cfDI were measured. Our results showed that the cfDI value increased gradually with the progression of NACT. At each checkpoint, the mean cfDI values of Alu were 0.51±0.26, 0.56±0.03, and 0.76±0.26 (P<0.01), respectively. The mean cfDI values of LINE1 were 0.23±0.03, 0.32±0.03, and 0.40±0.26 (P<0.05), respectively. Although the mean cfDNA concentration increased when patients finished their NACT, there was no statistical difference between the concentrations pre and post NACT, whether for Alu (75.89, 80.69, 142.26 ng/mL) or LINE1 (82.47, 77.89, 122.25 ng/mL) (Figure 1). In addition, CA153 expression before and after NACT was also assessed. The results showed that the average values of CA153 were 28.13, 25.11, and 24.46 ng/mL respectively, and there was no statistical difference.

Increased cfDI was associated with tumor shrinkage and Ki67 decline
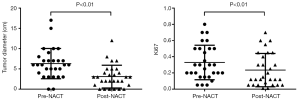
We measured the maximum diameter of each tumor pre and post NACT using ultrasound and found that the mean tumor diameter decreased significantly (from 6.31±3.73 to 3.10±2.80 cm, P<0.01). In addition, immunohistochemical analysis showed reduced Ki67 levels post NACT, which was expected because of the reduction in cellularity caused by NACT (from 32.93%±21.28% to 23.52%±20.52%, P<0.01) (Figure 2).
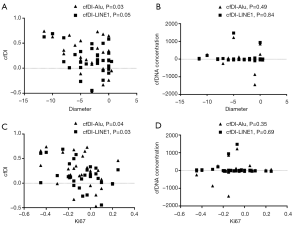
Correlation analysis showed that tumor shrinkage correlated positively with the increase in the cfDI value of Alu (P=0.03) and had a near statistical correlation with the cfDI value of LINE1 (P=0.05). However, the increase in the cfDNA concentration was not associated with tumor shrinkage. Similar results were obtained in the analysis of Ki67 levels. Ki67 levels correlated negatively with the cfDI values (Alu: P=0.04; LINE1: P=0.03); however, the relationship between the cfDNA concentration and Ki67 was not significant (Figure 3).
CfDI after NACT was inversely correlated with the number of metastatic lymph nodes
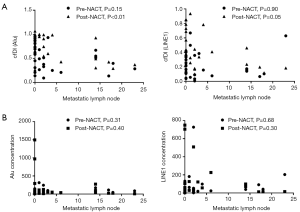
All patients eventually underwent surgery, and excised lesions and lymph nodes were examined pathologically. The results of the pathological examination showed that five patients had no residual tumor cells in their primary lesions. The average number of lymph nodes removed was 18.90 (range: 0 to 34), and the average number of positive lymph nodes was 3.79±6.54 (range: 0 to 23). No positive lymph nodes were found in 14 patients. In brief, four patients reached a pathologically complete response (pCR). No clear relationship was found between the pCR and the cfDNA concentration or cfDI. However, we found that the cfDI of Alu after NACT was significantly inversely correlated with the number of metastatic lymph nodes (P<0.01) and there was a near-significant inverse relationship between LINE1 cfDI and the number of metastatic lymph nodes (P=0.05) (Figure 4).
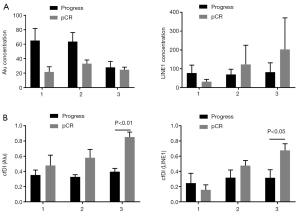
Patients with increasing cfDI tended to obtain better NACT responses
In this study, none of the four patients with pCR showed tumor recurrence during follow-up, while the other four patients showed distant metastasis within 6 months after surgery. We compared the difference in cfDI values between patients who achieved a pCR and those with metastasis. We found that patients who achieved a pCR had gradually increasing cfDI values during NACT; however, those showing disease progression after surgery demonstrated no significant change in cfDI values. Furthermore, at the end of the NACT, the mean cfDI value in the pCR group was significantly larger than that in the metastatic group (Figure 5).
Discussion
In the present study, we assessed the cfDNA concentration and cfDI of Alu and LINE1 genomic elements in patients with breast cancer receiving NACT. We found that these indicators had no significant correlation with the clinical and pathological characteristics of patients before NACT. The cfDI value increased significantly after treatment; however, the changes in the cfDNA concentration and CA153 were not significant. When patients received NACT, both tumor volumes and proliferative capacities decreased significantly. Correlation analysis showed that tumor shrinkage and reduced Ki67 levels correlated positively with an increased cfDI value. The results also suggested that the number of pathologically detected metastatic lymph nodes after surgery correlated inversely with the cfDI measured after NACT. Our results also suggested that a gradual increase in the cfDI during NACT might suggest a better treatment response. Conversely, if a patient’s cfDI did not change significantly, it might indicate a poor response and eventual recurrence.
Recently, many studies have been carried out to investigate the potential of the cfDNA concentration and cfDI as new tumor biomarkers. However, previous studies formed inconsistent conclusions concerning these two markers in patients with cancer (16-18). There are several partial explanations for this contradiction. First, the source of cfDNA is unclear. Some studies believed that an increased cfDI is associated with cfDNA derived from apoptotic and necrotic cells in patients with cancer, while it is derived only from apoptotic cells in healthy individuals (19,20). Apoptotic cells release DNA fragments that are usually 185–200 bp long, while DNA fragments from necrotic cells vary in size and can even be several kilobase pairs (21). Different sources have been proposed as the cause of the increased cfDI observed in patients with cancer (22). However, an increasing number of reports have shown that cfDNA in patients with cancer is highly variable and mainly comprises short DNA molecules (<200 bp), which preferentially carry tumor-associated gene aberrations (23). Our previous study also confirmed the presence of short DNA fragments, as well as decreased cfDI values, in patients with breast cancer compared with those with benign breast tumors (24).
Although the significance of the cfDNA concentration and cfDI in the diagnosis of cancer has been widely studied, the predictive and prognostic values of these biomarkers in patients receiving NACT have only been investigated in a small number of studies. Previous studies mainly identified a decreasing cfDNA level as a marker for early treatment response, e.g., in lung cancer (25) or rectal cancer during neoadjuvant therapy (26); however, cfDI has not yet been addressed for this indication. Lehner et al. reported that the kinetics of Alu levels during NACT showed decreases in patients with complete remission, while in patients who showed no change in their disease, increased Alu levels were observed (P=0.033). The authors also demonstrated that cfDI and CA153 were not informative for therapy outcome (27). The findings of the present study showed contradictory results to those of previous studies. Although there was no statistically significant change in the cfDNA concentration, it showed an increasing trend after NACT. We speculated that this could partly be attributed to the fact that the tumors destroyed by cytotoxic drugs could release large amounts of DNA molecules into circulation that exceeded the degradation rate of deoxyribonucleases. Besides, DNA molecules released by damaged or necrotic tumor cells trended to be long fragments, which would explain why the cfDI increased significantly after NACT. In addition, the number of metastatic lymph nodes correlated inversely with the cfDI in the blood of patients after NACT in this study. This phenomenon suggested that patients with breast cancer with lymph node metastasis often have a greater tumor burden, which might lead to an increase in shorter DNA fragments in blood and consequently lower cfDI values. However, this speculation requires confirmation by further research.
To some extent, our results confirmed the potential of cfDI to monitor the therapeutic response of breast cancer to NACT. However, some limitations were present. First, the sample size of this study was small, and it was a retrospective study. Randomized controlled studies with larger sample sizes are needed to further confirm the significance of cfDI in the background of NACT. Second, we did not further explore whether NACT regimens had effects on the cfDNA concentration or cfDI because of the small sample size. Finally, this was a preliminary study and the follow-up time was short. Although we showed some positive findings, the results were not sufficiently persuasive.
In summary, this study demonstrated that cfDI had the potential to be used as an indicator to monitor the therapeutic response of breast cancer to NACT. However, because of the limitations of this study, more research is needed to explore the clinical application of cfDI in the context of NACT.
Table S1
| Gene | Sequence (5'-3') |
|---|---|
| Alu (111 bp) | Forward: CTGGCCAACATGGTGAAAC, reverse: AGCGATTCTCCTGCCTCAG |
| Alu (260 bp) | Forward: ACGCCTGTAATCCCAGCA, reverse: CGGAGTCTCGCTCTGTCG |
| LINE1 (97 bp) | Forward: TGGCACATATACACCATGGAA, reverse: TGAGAATGATGGTTTCCAATTTC |
| LINE1 (266 bp) | Forward: ACTTGGAACCAACCCAAATG, reverse: CACCACAGTCCCCA GAGTG |
Table S2
| Number | Age (year) | Menopause | Tumor size (cm) | Axillary lymph node status | ER | PR | HER-2 | Ki67 | CA153 (ng/mL) | NACT regimen |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 43 | No | 9 | multiple suspicious lymph nodes | (+) | (+) | (+) | 20% | 62.82 | TEC ×3 |
| 2 | 41 | No | 3 | 1 suspicious lymph nodes, 2.0 cm × 2.0 cm | (+) | (−) | (−) | 60% | 19.28 | EC ×4-T ×4 (14 days) |
| 3 | 43 | No | 6 | 2 suspicious lymph nodes, 1.5 cm × 1.5 cm, 2.0 cm × 1.5 cm | (−) | (−) | (+) | 35% | 52.74 | EC ×4-T ×4 (14 days) |
| 4 | 43 | No | 5 | no obvious abnormal lymph nodes | (+) | (+) | (−) | 15% | 22.55 | EC ×4-T ×4 (14 days) |
| 5 | 42 | No | 15 | 1 suspicious lymph nodes, 1.8 cm × 1.5 cm | (−) | (−) | (−) | 70% | 9.35 | EC ×4-T ×4 (14 days) |
| 6 | 32 | No | 10 | 2 suspicious lymph nodes, 2.0 cm × 1.5 cm, 1.8 cm × 1.5 cm | (−) | (−) | (+) | 50% | 37.25 | TCH×3 |
| 7 | 60 | Yes | 10 | 1 suspicious lymph nodes, 3.0 cm × 2.0 cm | (−) | (−) | (+) | 80% | 86.15 | TEC ×4 |
| 8 | 29 | No | 3 | no obvious abnormal lymph nodes | (−) | (−) | (+) | 10% | 9.55 | EC ×4-TH×4 |
| 9 | 47 | No | 17 | 1 suspicious lymph nodes, 2.0 cm × 1.5 cm | (−) | (−) | (+) | 20% | 9.18 | EC ×4-TH×4 |
| 10 | 44 | No | 5 | multiple suspicious lymph nodes | (−) | (−) | (+) | 60% | 25.88 | TEC ×3 |
| 11 | 41 | No | 4 | 1 suspicious lymph nodes, 2.0 cm × 1.0 cm | (+) | (+) | (+) | 25% | 33.21 | TEC ×3 |
| 12 | 52 | Yes | 10 | no obvious abnormal lymph nodes | (−) | (−) | (+) | 30% | 20.68 | EC ×4-T ×4 (14 days) |
| 13 | 64 | Yes | 7 | no obvious abnormal lymph nodes | (−) | (−) | (−) | 50% | 18.07 | FEC ×3 |
| 14 | 49 | No | 5 | 2 suspicious lymph nodes | (−) | (−) | (−) | 5% | 65.55 | FEC ×4 |
| 15 | 38 | No | 7 | no obvious abnormal lymph nodes | (−) | (−) | (+) | 30% | 43.89 | TEC ×3 |
| 16 | 36 | No | 6 | no obvious abnormal lymph nodes | (+) | (+) | (+) | 15% | 17.48 | TEC ×3 |
| 17 | 30 | No | 8 | no obvious abnormal lymph nodes | (−) | (−) | (+) | 50% | 22.05 | EC ×4-T ×4 (14 days) |
| 18 | 47 | No | 6 | 1 suspicious lymph nodes, 2.0 cm × 1.0 cm | (+) | (+) | (−) | 5% | 21.69 | FEC ×3 |
| 19 | 49 | No | 3 | no obvious abnormal lymph nodes | (+) | (+) | (+) | 20% | 8.15 | FEC ×3 |
| 20 | 33 | No | 7 | no obvious abnormal lymph nodes | (−) | (−) | (+) | 20% | 33.25 | TEC ×3 |
| 21 | 61 | Yes | 7 | multiple suspicious lymph nodes | (+) | (+) | (+) | 45% | 76.16 | EC ×4-TH×4 |
| 22 | 45 | No | 6 | 1 suspicious lymph nodes, 1.5 cm × 1.2 cm | (+) | (+) | (−) | 70% | 9.42 | EC ×4-TH×4 |
| 23 | 30 | No | 3 | no obvious abnormal lymph nodes | (+) | (+) | (+) | 5% | 9.29 | EC ×4-TH×4 |
| 24 | 34 | No | Diffuse mass | 2 suspicious lymph nodes, 1.6 cm × 1.2 cm, 1.0 cm × 1.0 cm | (+) | (−) | (+) | 30% | 22.87 | TEC ×3 |
| 25 | 67 | Yes | 5 | 1 suspicious lymph nodes, 2.2 cm ×1.8 cm | (−) | (−) | (+) | 30% | 22.11 | EC ×4-TH×4 |
| 26 | 52 | No | Diffuse mass | multiple suspicious lymph nodes | (−) | (−) | (+) | 50% | 7.22 | FEC ×3 |
| 27 | 59 | Yes | 7 | multiple suspicious lymph nodes | (+) | (+) | (−) | 15% | 24.65 | TEC ×8 |
| 28 | 52 | No | 3 | 2 suspicious lymph nodes, 2.8 cm × 2.0 cm, 1.8 cm × 1.5 cm | (+) | (+) | (−) | 20% | 12.04 | TEC ×8 |
| 29 | 55 | Yes | 6 | no obvious abnormal lymph nodes | (+) | (+) | (+) | 20% | 13.13 | FEC ×3 |
TEC ×3, TEC ×4, TEC ×8: 3, 4 or 8 cycles of docetaxel/epirubicin/cyclophosphamide; EC ×4-T ×4 (14 days): 8 cycles of epirubicin/cyclophosphamide followed by paclitaxel every 2 weeks; EC ×4-TH ×4: 8 cycles of epirubicin/cyclophosphamide followed by paclitaxel plus trastuzumab; FEC ×4, FEC ×3: 3 or 4 cycles of fluorouracil/epirubicin/cyclophosphamide; TCH ×3: 3 cycles of docetaxel/carboplatin/trastuzumab. All patients were expected to complete all chemotherapy before surgery. However, some patients strongly required early operation only after 3–4 cycles due to excessive psychological pressure; Some patients were reluctant to continue chemotherapy because of severe side effects; and since this is a retrospective study, it was unknown exactly why individual patients did not complete standard neoadjuvant chemotherapy. But look at the medical records, we found that most of the patients had completed the rest of the chemotherapy after the surgery.
Table S3
| Variable | Alu conct | cfDI-Alu | LINE1 conct | cfDI-LINE1 | CA153 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 95% CI | P | 95% CI | P | 95% CI | P | 95% CI | P | 95% CI | P | |||||
| Age | −3.82 to 6.45 | 0.60 | −0.02 to 0.01 | 0.72 | −6.42 to 12.60 | 0.51 | −0.02 to 0.00 | 0.41 | −1.21 to 1.88 | 0.66 | ||||
| Menopause | −80.33 to 155.67 | 0.51 | −0.36 to 0.37 | 0.97 | −129.69 to 307.35 | 0.41 | −0.25 to 0.29 | 0.87 | −39.79 to 31.31 | 0.81 | ||||
| ER | −177.47 to 78.88 | 0.43 | −0.76 to 0.03 | 0.07 | −251.67 to 223.04 | 0.90 | −0.41 to 0.18 | 0.42 | −42.58 to 34.65 | 0.83 | ||||
| PR | −108.61 to 158.29 | 0.70 | 0.04 to 0.86 | 0.04 | −174.24 to 320.02 | 0.55 | −0.12 to 0.49 | 0.23 | −37.72 to 42.70 | 0.90 | ||||
| HER-2 | −66.34 to 80.97 | 0.84 | −0.24 to 0.22 | 0.94 | −64.29 to 208.50 | 0.28 | −0.32 to 0.01 | 0.07 | −12.57 to 31.81 | 0.38 | ||||
| Ki67 | −250.68 to 79.42 | 0.29 | −0.66 to 0.36 | 0.54 | −353.21 to 258.08 | 0.75 | −0.31 to 0.45 | 0.70 | −37.26 to 62.19 | 0.61 | ||||
| Tumor size | −8.44 to 11.81 | 0.73 | −0.05 to 0.00 | 0.14 | −10.62 to 26.88 | 0.38 | −0.03 to 0.02 | 0.85 | −3.15 to 2.95 | 0.95 | ||||
ER, estrogen receptor; PR, progesterone receptor; HER-2, human epidermal growth factor receptor-2; conct, concentration.
Acknowledgments
Funding: This work was supported in part by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.08.05). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethical and scientific committee of the institution (2010-SR-091). Informed consent was obtained from all participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- DeSantis CE, Fedewa SA, Goding Sauer A, et al. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA Cancer J Clin 2016;66:31-42. [Crossref] [PubMed]
- Harbeck N, Gnant M. Breast cancer. Lancet 2017;389:1134-50. [Crossref] [PubMed]
- Zardavas D, Piccart M. Neoadjuvant therapy for breast cancer. Annu Rev Med 2015;66:31-48. [Crossref] [PubMed]
- DeMichele A, Yee D, Esserman L. Mechanisms of Resistance to Neoadjuvant Chemotherapy in Breast Cancer. N Engl J Med 2017;377:2287-9. [Crossref] [PubMed]
- Rigter LS, Loo CE, Linn SC, et al. Neoadjuvant chemotherapy adaptation and serial MRI response monitoring in ER-positive HER2-negative breast cancer. Br J Cancer 2013;109:2965-72. [Crossref] [PubMed]
- Penault-Llorca F, Radosevic-Robin N. Biomarkers of residual disease after neoadjuvant therapy for breast cancer. Nat Rev Clin Oncol 2016;13:487-503. [Crossref] [PubMed]
- Crowley E, Di Nicolantonio F, Loupakis F, et al. Liquid biopsy: monitoring cancer-genetics in the blood. Nat Rev Clin Oncol 2013;10:472-84. [Crossref] [PubMed]
- Hanash SM, Baik CS, Kallioniemi O. Emerging molecular biomarkers--blood-based strategies to detect and monitor cancer. Nat Rev Clin Oncol 2011;8:142-50. [Crossref] [PubMed]
- Schwarzenbach H, Hoon DS, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer 2011;11:426-37. [Crossref] [PubMed]
- Schwarzenbach H. Circulating nucleic acids as biomarkers in breast cancer. Breast Cancer Res 2013;15:211. [Crossref] [PubMed]
- Zonta E, Nizard P, Taly V. Assessment of DNA Integrity, Applications for Cancer Research. Adv Clin Chem 2015;70:197-246. [Crossref] [PubMed]
- Heidary M, Auer M, Ulz P, et al. The dynamic range of circulating tumor DNA in metastatic breast cancer. Breast Cancer Res 2014;16:421. [Crossref] [PubMed]
- Diehl F, Schmidt K, Choti MA, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med 2008;14:985-90. [Crossref] [PubMed]
- Deligezer U, Eralp Y, Akisik EZ, et al. Effect of adjuvant chemotherapy on integrity of free serum DNA in patients with breast cancer. Ann N Y Acad Sci 2008;1137:175-9. [Crossref] [PubMed]
- Madhavan D, Wallwiener M, Bents K, et al. Plasma DNA integrity as a biomarker for primary and metastatic breast cancer and potential marker for early diagnosis. Breast Cancer Res Treat 2014;146:163-74. [Crossref] [PubMed]
- Umetani N, Giuliano AE, Hiramatsu SH, et al. Prediction of breast tumor progression by integrity of free circulating DNA in serum. J Clin Oncol 2006;24:4270-6. [Crossref] [PubMed]
- Wang BG, Huang HY, Chen YC, et al. Increased plasma DNA integrity in cancer patients. Cancer Res 2003;63:3966-8. [PubMed]
- van der Vaart M, Pretorius PJ. The origin of circulating free DNA. Clin Chem 2007;53:2215. [Crossref] [PubMed]
- Jin Z, El-Deiry WS. Overview of cell death signaling pathways. Cancer Biol Ther 2005;4:139-63. [Crossref] [PubMed]
- Jahr S, Hentze H, Englisch S, et al. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res 2001;61:1659-65. [PubMed]
- Deligezer U, Eralp Y, Akisik EE, et al. Size distribution of circulating cell-free DNA in sera of breast cancer patients in the course of adjuvant chemotherapy. Clin Chem Lab Med 2008;46:311-7. [Crossref] [PubMed]
- Rykova EY, Morozkin ES, Ponomaryova AA, et al. Cell-free and cell-bound circulating nucleic acid complexes: mechanisms of generation, concentration and content. Expert Opin Biol Ther 2012;12:S141-53. [Crossref] [PubMed]
- Wang W, Liang M, Ma G, et al. Plasma cell-free DNA integrity plus circulating tumor cells: a potential biomarker of no distant metastasis breast cancer. Neoplasma 2017;64:611-8. [Crossref] [PubMed]
- Gautschi O, Bigosch C, Huegli B, et al. Circulating deoxyribonucleic Acid as prognostic marker in non-small-cell lung cancer patients undergoing chemotherapy. J Clin Oncol 2004;22:4157-64. [Crossref] [PubMed]
- Zitt M, Muller HM, Rochel M, et al. Circulating cell-free DNA in plasma of locally advanced rectal cancer patients undergoing preoperative chemoradiation: a potential diagnostic tool for therapy monitoring. Dis Markers 2008;25:159-65. [Crossref] [PubMed]
- Lehner J, Stotzer OJ, Fersching D, et al. Circulating plasma DNA and DNA integrity in breast cancer patients undergoing neoadjuvant chemotherapy. Clin Chim Acta 2013;425:206-11. [Crossref] [PubMed]