
MRgFUS for liver and pancreas cancer treatments: the Umberto I hospital experience
Primary cancer of the exocrine pancreas is one of the leading causes of mortality worldwide and it represents the fourth cause of cancer-related death in United States and Western Regions (1,2). At present, incidence rate equals mortality rate (3-5) and, According to the American Cancer Society Statistics (1), the 5-year overall survival rate of patients with pancreatic adenocarcinoma is 6%, registering a slightly increase from the 2% of 1975-1977 (6). In the last few years several studies were conducted to obtain more insights into this cancer (7), to improve early diagnosis and to identify new therapeutic strategies to improve quality of life and survival time (7-9). Nevertheless, at present, surgery remains the only option to eradicate pancreatic cancer, but it is feasible in approximately 20% of patients due to invasion of local structures or to metastases in other organs at the time of diagnosis (10), while the prognosis of patients in which surgery is not amenable is still miserable even combining all the proved therapeutic interventions (11-13). New therapeutic modalities have been introduced in recent years, such as radiofrequency, laser ablation, microwaves, and cryo-ablation therapies to provide additional therapeutic options when conventional therapies fail or are not applicable in a broad-spectrum of disease (14). Notwithstanding the successful of these methods to treat other tumours, these procedures are not yet envisaged by current treatment guidelines for pancreatic cancer, probably due to the significant risk of collateral damages to neurovascular structures or due to risk of massive pancreatitis (15,16).
Likewise, liver cancer is among the commonest malignant neoplasms worldwide and it accounts for more than 600,000 new cases every year (1,17). It is the sixth most common cancer worldwide and the third most common cause of cancer-related deaths (1,18). Although most cancers in liver are metastatic lesions, hepatocellular carcinoma (HCC) is the most common of primary liver cancer accounting for more than 90% of primary neoplasms (19,20). Surgery is the cornerstone in the management of patients with HCC, however only few patients are eligible for hepatic resection upon diagnosis and, moreover, recurrence is common with a 5-year recurrence rate of over 50% (21-23). Liver transplantation can provide an ultimate treatment but liver grafts are insufficient and not all patients with HCC can be transplanted because of tumour’s or general characteristics (24). In the last decade local ablative therapies like radiofrequency ablation (RFA) and transarterial chemoembolization (TACE) led a revolution in the treatment algorithm for HCC (25-30). However, RFA may not be well tolerated in patients with cirrhosis, severe complications may occur after prolonged ablation (31) and 2-year survival rate after TACE is only around 30% (30,32).
High intensity focused ultrasound (HIFU) is a totally non-invasive and extracorporeal technique that has recently emerged as a new ablative therapy for both primary solid tumours and metastatic disease (33,34). The ultrasound beams used in HIFU deliver mechanical energy required to raise the target tissue temperature to a cytotoxic level (60/80 °C), inducing a coagulative necrosis, through a short lasting thermal stimulation (10/30 seconds) and can be focused at a distance from the therapeutic transducer (35-39). HIFU ablation with US guidance (USgFUS) has been widely validated for the local treatment of tumours in abdominal organs (39-41), becoming an accepted clinical alternative to conventional ablative techniques in patients with both liver and pancreatic cancers (42-44). Magnetic resonance imaging (MRI) guided focused ultrasound surgery (MRgFUS) is a newly introduced treatment that combines MR imaging for precise targeting and monitor with a tumour-ablative apparatus (HIFU). MRgFUS recently received FDA and CE approval for clinical treatment of uterine fibroids and bone tumours (45) and it is, actually, under evaluation through preclinical or clinical trials (46) for other applications. MRI offers significant advantages in the guidance of thermal ablation of focused US, such as high-resolution imaging in any orientation for treatment planning and, most important, the ability to create quantitative temperature maps (47). MRI thermometry, indeed, is the only technique that can control the energy deposition on the ablation target, monitoring also thermal effects on surrounding tissues (47). Beside these advantages, MRgFUS is facing decisive challenges in treatment of abdominal organs due to organ movements during the ablation (42,48).
In this clinical scenario our research group at Sapienza University of Rome - Umberto I Hospital performed a preliminary study to evaluate feasibility and safety of MRgFUS for treatment of selected pancreatic and liver tumours (49,50) and we are still enrolling patients to evaluate pain palliation in patients with pancreatic adenocarcinoma and potential decrease in tumor growth or tumor. The aim of this paper is to review the results of our experience and to discuss limitations and future perspectives of this technique.
Materials and methods
Patient selection, treatment feasibility and endpoints
Our preliminary study received institutional review board approval and all the enrolled patients signed a dedicated informed consent. Inclusion criteria were as follow: biopsy-proven unresectable primary liver or pancreatic cancer, failure or refusal to control tumour growth and/or symptoms by conventional treatment. Exclusion criteria were: distant metastases, life expectancy less than 3 months, contraindication to general anaesthesia or contrast-enhanced MR. At the time of enrolment patients with pancreatic adenocarcinoma were treated with a standard chemotherapy regimen since at least 3 months before the MRgFUS treatment and underwent unsuccessful radiotherapy for pain palliation at least 1 month before MRgFUS; all patients continued the CHT after the procedure during the follow-up period.
Tumour identification and staging were performed with both CT and MR. CT examinations were performed using a 64-slice scanner (Somatom Definition, Siemens, Erlangen, Germany) before and after the intravenous administration of 0.5 mL/kg of an iodinated contrast agent (Iomeron 400, Bracco SpA, Milan, Italy) that contains iodine at a high concentration of 400 mgI/mL. Both pancreatic and portal venous phase images were acquired. MR examinations were performed on a 3T scanner (Discovery MR750, GE Medical Systems, Milwaukee, Wisconsin, USA) with a combination of T1- and T2-weighted morphological sequences acquired with and without spectral saturation of the fat signal, as well as dynamic 3D contrast-enhanced T1-weighted sequences acquired after the administration of 0.5 mL/kg of gadobenate dimeglumine (MultiHance, Bracco SpA, Milan, Italy).
CT and MR were used to assess feasibility of MRgFUS treatment. Patients were considered eligible for MRgFUS according to the following criteria:
- Lesion located in easily accessible portion of the pancreas (body/isthmus);
- Lesion visible on both MR and CT images;
- Lesion not shielded by extensive scarring, metal clips, hollow viscera or bone.
Patients were considered ineligible for MRgFUS in presence of one or more of the following reasons:
- General contraindications to MR or to the administration of iodinated and/or gadolinium based contrast agents;
- Target lesion located in the deep head portion of the pancreas or in the pancreatic tail due to bowel loop interposition;
- Extensive scarring along the energy path of the planned treatment area;
- Pre-existing acute or chronic medical conditions (including cardiovascular, respiratory, neurologic or infectious diseases) which would impede anaesthesia or overall treatment.
Clinical endpoints in patients with pancreatic adenocarcinoma were pain control and palliative tumour ablation; meanwhile in patients with HCC, endpoint was complete tumour ablation.
Patient positioning and pre-treatment imaging
All procedures were performed on 3T MR scanner (GE medical systems), featuring a 208 element annular phased-array HIFU transducer embedded into the patient table (ExAblate 2100, InSightec, Haifa, Israel, diameter: 120 mm; radius of curvature: 160 mm; focal distance: 60-200 mm; frequencies: 0.95-1.35 MHz; and energy range 100-7,200 J) by an experienced abdominal radiologist and certified MRgFUS operator (A.N.—12 years of experience in abdominal imaging, 5 years of experience in MRgFUS). Treatments were performed under general anaesthesia with patients placed in prone position. An intestinal preparation of 12 h was observed by all patients to reduce the distension of stomach, small bowel, and colon. A convex gel pad was used to compress the abdominal wall, to displace viscera, to minimize distance between transducer and target and to achieve an optimal acoustic window avoiding air and gas interference. An anaesthesiologist controlled the amount of air to be inhaled by the patient and the duration of the apnoea, using an MR-compatible respiratory monitoring system with a mechanical ventilator (iVent201), as previously for USgFUS ablation of liver and pancreatic tumours (51-53). A combination of T1-w, T2-w, and DWI sequences were acquired and repeated three times to confirm organ shift and lesion position in all planes in the expiratory phase of controlled respiration. Before starting the planning, 3D GRE T1-w contrast-enhanced sequences (0.5 mL/kg of gadobenate dimeglumine—MultiHance, Bracco SpA, Milan, Italy) were acquired on three-planes to define the target area correctly.
Treatment planning, ablation and post-procedural care
Based on images acquired with patients lying in treatment position, the operator plans the treatment. First of all the operator chooses and confirms the initial position and orientation of the transducer with respect to the target lesion. Then the operator performs a manual segmentation of the region of treatment (ROT) including a margin of 4-5 mm tumour-free tissue into the ablation area. After the drawing of the ROT, treatment plan proceeds with the identification of limited energy density regions (LEDR), including skin surface and bowel, in order to limit the energy dispersion outside the ROT. The system software automatically calculated the optimal ablation coverage of the lesion with a minimal number of sonications, taking into account the defined LEDRs to protect adjacent tissues; this automated treatment plan was adjustable by the operator in every aspect, including the sonication’s locations and numbers, the energy levels, the sonication’s duration and the spot’s sizes. After defining ROT and LEDRs, a low-energy (350-400 J, 1.10 MHz) test sonication is performed to confirm the path and to correct the direction of the ultrasound beam into the ROT for calibration purpose, avoiding ribs and bowel. Then the treatment stage began using the full-energy sonication routine, with a temperature threshold of 65° to define successful ablation in the ROT. Real-time quantitative MR thermometry is obtained using a phase-difference fast spoiled gradient-echo sequence that provides temperature-dependent images in real-time, substantially independent from tissue type and thermally induced tissue changes. Thermometry (54) was used to evaluate temperature variations in the target area and surrounding tissues, and to provide a closed-loop control of energy deposition, with temperature accuracy of 1 °C, spatial resolution of 1 mm, and temporal resolution of 3 seconds. Temperature-sensitive MR images were obtained following each sonication to estimate tissue temperature and ablated volume. In order to reduce movement, ablation was performed during controlled deep inspiration, at the same extent at which the morphological imaging sequences had been acquired. Treatment was considered complete once the lesion and 5-mm of tumour-free margins have been completely ablated.
Contrast-enhanced T1-w sequences were acquired immediately after treatment to verify the effects of the ablation on the target lesion and to exclude thermal damage to surrounding structures. Immediately after treatment skin was evaluated on site to identify skin burns or other forms of thermal damages and then patients were hospitalized for 24 hours to monitor adverse effects. All the patients received e.v. infusion of steroids (40 mg methylprednisolone) to avoid vascular compression by the intra- and extra-lesion edema to prevent portal thrombosis (33). During the 24 hours of hospitalization we monitored vital signs (oximetry, electrocardiography, blood tests), pancreatic functionality (to detect early signs of pancreatitis), on-demand drug administration (analgesics and/or antiemetics) and morphine infusion via a disposable elastomeric pump.
Evaluation of treatment outcome
Treatment success was assessed by a clinical point of view in terms of pain palliation while tumour control was assessed according to imaging findings. Clinical criteria were primarily based on changes in the visual analog pain score (VAS), a Likert-type scale with values between 0 and 10 to express pain severity, and, secondarily, on changes in the drug schedule, evaluated at 1, 3, 7, 14 days and, thereafter, at 30-day intervals following treatment. Imaging evaluations were performed immediately after ablation and, therefore, at 3 and 6 months of follow-up by two experienced abdominal radiologists (M.A.—8 years of experience, and B.C.M.—6 years of experience) in consensus. Treatment effects were assessed according to qualitative changes in tissue density/intensity and contrast enhancement on both CT and MR that were consistent with tumour necrosis; recurrence in the ablated area was assessed as well. A third independent radiologist (F.Z.—5 years of experience in abdominal imaging) performed a quantitative analysis on 3D contrast-enhanced T1-w MR sequences to obtain non-perfused volume (NPV). NPV was defined as the volume of cancer tissue enhancing at baseline that did not show any contrast uptake after treatment and it was used as a quantitative indicator of local tumour control. The procedure was considered successful if at least 50% of the ablated area was considered overlapping with NPV at follow-up.
Statistical analysis
Statistical analysis was performed using dedicated software (SPSS Statistics 19 for Macintosh; IBM Company, Armonk, NY, U.S.A. and Stata SE 12 for Macintosh, StataCorp, Lakeway Drive, Texas, USA). All continuous values were expressed as mean ± SD [95% confidence interval (CI)], qualitative values were expressed as n (%). Differences in NPV values between baseline and follow-up examinations were tested using the analysis-of-variance (ANOVA) for repeated-measures and paired-sample T-test as appropriate in relation to the number of tested observations, repetition of measurements and data distribution. The Wilcoxon signed-rank test was used to test differences in VAS’ values between pre- and immediately post-treatment interviews; overall differences between pre- and follow-up examinations were tested using ANOVA for repeated-measures. Spearman’s correlation coefficient was used to verify correlation between the NPV values and VAS changes immediately after treatment. A P value <0.05 was considered statistically significant for all tests.
Results
We enrolled seven patients (five males, two females, average age 67±5 years) with biopsy proven, TNM stage III, unresectable adenocarcinoma of the pancreatic body, and one patient (female, 64 years) with atypical, biopsy-proven hypovascular HCC in liver segment VI. Six patients with pancreatic cancer underwent MRgFUS treatment; a single case (1/7, 14.3%) was excluded due to the interposition of the transverse colon between the transducer and the target area that inhibits a safe ablation. MRgFUS treatment for patients with pancreatic adenocarcinoma was performed 30±5 days after the initial diagnosis; patient with HCC was already waiting for a liver transplant. Treatment details are shown in Table 1. All patients were monitored for adverse events and discharged uneventfully the day after treatment.
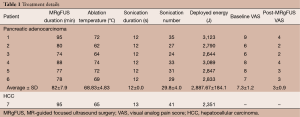
Full table
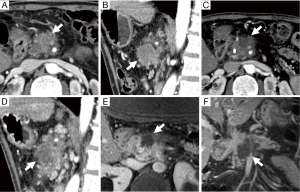
From a clinical point of view, all the patients with pancreatic adenocarcinoma experienced symptom relief after treatment. There was indeed a significant decrease of VAS’ values from 7±1 at baseline to 3±1 one week after treatment (P=0.017). During the sequent follow-up evaluations no significant changes were detected and analgesic medications were discontinued in all patients. Pancreatic adenocarcinomas mean volume at baseline was 20±5.66 mL (95% CI, 13.8-29.1). Vascular encasement (celiac artery, superior mesenteric artery and/or portal vein) was present in all patients. Follow-up CT and MR imaging as well as NPV demonstrated technical success in all patients in terms of local tumour control. In details, tumour necrosis was qualitatively identified in all cases; mean NPV was of (60±5)% [mean tumor volume at baseline: 20±5.6 mL; mean NPV at 6-month follow-up: (12.9±4.6)%] of the ablated area, without tumour re-growth in five cases (Figure 1). In a single case CT and MR at the 6-month follow-up demonstrated a small cluster of solid, enhancing tissue at the periphery of the ablated area. Spearman’s correlation coefficient demonstrates a positive, statistically significant correlation between NPV and VAS after MRgFUS treatment (rho=0.87; P=0.000). The tumour volume remained essentially stable over-time with mean volume of 21 and 24 mL, at 3 and 6 months respectively (P>0.05); in a single patient the overall tumour volume increased (17.7 mL at baseline; 30 mL at 6 months) due to peripheral tissue growth while the NPV core remained stable (13.2 and 12.9 mL at 3 and 6 months, respectively).
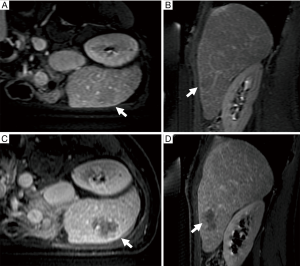
The patient with HCC showed complete ablation of the target area without residual viable tissue (100% NPV) both at immediate post-treatment MR than at 1-month follow-up examination (Figure 2). Subsequent follow-up at 3- and 6-month showed a small focus (8 mm in maximum diameter) of recurrent tumour tissue along the lateral edge of the ablation zone, with a NPV of 85%. Sixteen months after MRgFUS, the patient underwent liver transplantation; histopathology demonstrated coagulation necrosis of ablated area with a focus of tumour regrowth along the ablation margins.
Discussion
Background
The ideal treatment of a solid cancer should achieve tumour cell death without damage to the adjacent normal tissues. Accordingly, in the last few decades percutaneous techniques have achieved wide-spread acceptance in medical community also due to significant technological improvements and reduced complication rates of these techniques. At present, RFA, transcatheter arterial chemoembolization, percutaneous ethanol injection, cryoablation, microwave coagulation, laser-induced interstitial thermotherapy, and HIFU, have been used to ablate solid tumours and are still spreading throughout daily clinical routine. However, HIFU is slightly different from the others, being the only one to be completely extracorporeal and incision less. Moreover the coupling of this totally non-invasive technique with the capability of real-time granted by US guidance led to an increasing interest for this technique in the management of solid tumours in abdominal organs (48). However, despite the advantages of US guidance, USgFUS is performed without real-time assessment of thermal damage leading to a possible suboptimal lesion necrosis and need of re-treatment. Moreover, US has some issues related to intrinsic limitations of the technique itself that can limit the accessibility of pancreatic lesions thereby compromising treatment (55). In this clinical scenario, the MR guidance completely overcomes these limitations, allowing detailed morphological evaluation of both the target lesion and the adjacent anatomic areas throughout all treatment stages, enabling, moreover, real-time monitoring of temperature and, therefore, estimation of thermal damage.
Pancreatic adenocarcinoma
Pancreatic adenocarcinoma is often inoperable at time of diagnosis, therefore palliative therapy for pain relief is mandatory and the development of effective local therapies will likely be essential for this purpose. However pancreatic lesions still represent a challenge in terms of accessibility and ablation, due to the highly complex neurovascular anatomy of the region and to the risk of potentially severe collateral damages to surrounding structures with gastrointestinal bleeding, hollow viscera perforation and massive necrotizing pancreatitis (16). With the advent and sequent diffusion of USgFUS, initial successful experience in the treatment of pancreatic lesions in swine and in porcine model were reported without any significant adverse effects such as skin burns or evidence of pancreatitis suggesting that HIFU treatment for pancreatic cancer may be feasible and safe (56,57).
In 2011 Khokhlova (58) published a review analysis considering all the papers published in period 2000-2011. The first record of USgFUS used for palliative treatment of pancreatic cancer is an open-label study performed in China in 251 patients with advanced pancreatic cancer (TNM stages II–IV) (59). In this study USgFUS resulted in significant pain relief in 84% of the patients and, moreover, a significant reduction of volume was achieved in some cases without any significant adverse effects or pancreatitis, resulting in prolonged survival. After this breakthrough in pancreatic adenocarcinoma management several nonrandomized studies have investigated the potential role of USgFUS. Mostly were conducted in China and provided additional evidences to confirm that USgFUS can provide pain palliation without significant adverse effects (33,55,59-64). Actually the adverse effects reported were mild pancreatitis in two cases from Wang et al. (61); few skin burns, subcutaneous sclerosis and a pancreatic pseudo cyst reported by Xiong et al. (62) and a single case of portal vein thrombosis reported by Orsi et al. (33) Beside these adverse effects, pain relief was successfully achieved in almost all the studies with success percentage of almost 66.7% and, although it is still unclear, has been hypothesized that pain relief results from thermal damage to the nerve fibres in the tumour. Moreover in two studies USgFUS was used in combination with systemic chemotherapy (gemcitabine), leading to similar results in terms of pain relief and safety, but suggesting also a survival benefit (63,65).
In our opinion, MRgFUS is the natural evolution of USgFUS. The MR guidance could overcome the technical limits of USgFUS and therefore it could represent a more accurate, non-invasive ablation modality. Our experience is the first one to be reported in terms of treatment of advanced pancreatic adenocarcinoma using MRgFUS (49,50). Although the inclusion criteria we adopted in our study were strict, MRgFUS was performed successfully in all the enrolled patients but one excluded due to bowel interposition. There were no adverse effects after treatment and all patients were discharged uneventfully the day after confirming the safety of MRgFUS.
To overwhelm problems related to breathing movements, we performed our treatment using general anaesthesia and controlled respiration during the ablation cycle. This approach is feasible and adequate to treat pancreas without significant errors in targeting. This finding has a key role since lesion targeting in upper abdomen is one of the main challenges in MRgFUS due to the need to continuously track targeted lesions despite breath- and peristalsis-induced movements in the absence of a built-in monitoring device for respiratory gating. The retroperitoneal location of the organ contributed to the success of this approach as well as the adoption of the gel-pad cushion that displaces abdominal viscera from the pancreatic surface. Controlled respiration helps to limit also another significant issue in MRgFUS of upper abdominal, indeed movements can cause artefacts in thermometry measurements that may prevent correct assessment of tissue damage (66). However, despite the efficacy of our method, real-time motion tracking devices for precise targeting of the ultrasound beam in abdominal moving organs are currently under development, both from vendors and technological consortia (67) (FUSIMO, Collaborative Project, funded under the EU’s Seventh Framework Programme for Research and Technological Development. Work programme topics addressed: ICT-2009.5.3), in order to overcome movement-related limitations, mostly with regards of liver and kidney tumours’ treatment.
According to clinical results, in our cohort MRgFUS treatment was adequately successful for pain management: VAS values revealed a statistically significant and stable decrease of pain severity after ablation, without relevant recurrence at follow-up. This leads to a significant decrease in analgesic consumption immediately after treatment. As already hypothesized for USgFUS, pain relief could be probably related to the thermal damage of the peripheral fibres of the celiac plexus encased by tumour growth, in line with the pain modulation effects described for MRgFUS in several other applications, including spinal rhizotomy (68), bone metastases (69) and periosteal ablation in patients with symptomatic osteoid osteoma (70).
Our study demonstrates also a positive result in terms of induction of tumour necrosis. Indeed in all the patients the targeted area was successfully ablated with post-treatment images consistent with tissue necrosis as already demonstrated by other research group (71). There is a regrowth of tumour tissue in a single case 6 months after therapy, but it didn’t extend beyond the outer borders of the originally ablated lesion. However one of the greatest advantage of MRgFUS over almost all the other percutaneous technique is the chance to re-ablate recurrence since MRgFUS can be repeated several times on the same target without significant side effects.
Another major advantage of MRgFUS is the safety. It has been quite well demonstrated that USgFUS has only minor adverse effects (58) however in our small sample experience we registered neither minor nor major complications or adverse events related to the physical effects of treatment. MR guidance has a key role in this achievement thanks to the real-time control of energy deposition and therefore the chance to correct treatment while proceedings. Moreover, none of our patients demonstrated clinical or laboratory signs of pancreatitis after treatment, which is in agreement with what has already been demonstrated for US-guided HIFU ablation of pancreatic cancer (55). The absence of pancreatitis is probably related to a non-lytic cell death with degradation and inactivation of pancreatic enzymes; a phenomenon, known as thermal fixation. Thermal fixation is probably due to the relatively low temperature used in both MRgFUS and USgFUS as compared to the other ablation techniques and this could play a major role in preventing post-treatment pancreatitis. MR guidance allows also extremely precise planning preserving vessels from injury. Even in case of major vessels encasement, perivascular ablation can be performed safely without damages to vascular vessels leading to another major advantage of MRgFUS over the other percutaneous technique.
The preliminary results obtained in our study are promising in terms of both pain relief and palliation of local tumor growth however this study has several limitations that need to be addressed. First of all in our cohort all the tumours were located in the pancreatic body. While we choose this location for safety reason, we have not demonstrated device-accessibility of deep head or tail lesions. Another limitation arises from the inclusion criteria, the lack of histological support. According to advanced stage of selected patients, our patients have not underwent surgery after MRgFUS hence we have no histological data to demonstrate thermal damage, however the effects of thermal damage were accurately demonstrated by post-treatment imaging, with findings that are similar to those observed after other ablation procedures (72). Moreover HIFU induced thermal damage has been extensively demonstrated in uterine fibroids, bone, breast and prostate cancer (69,70).
Hepatocellular carcinoma (HCC)
HCC represents a problem of crescent interest worldwide. At present surgery still remains the cornerstone for management of these patients however only few patients are eligible for hepatic resection upon diagnosis (21-23). Beside resection, liver transplantation is the other definitive solutions but the shortage of deceased-donor liver grafts makes it impossible for everyone in need of this treatment. Bridging therapies, specifically intended to slow down tumour progression, like RFA and TACE led a revolution in the treatment algorithm for HCC however are not well tolerated in all patients and recurrence rate is still too high (25-32).
In the last few years HIFU has been proved to be safe and effective in patients with HCC improving also the quality of life. Xu et al. demonstrated symptoms improvement and pain relief in 84.8% of the 145 patients they treated with a 2-year survival rate from 46.5% in stage IIIa patients to 80% of stage Ib patients (73). Ng et al. treated 49 patients with unresectable HCC demonstrating technical success in 79.5% of cases with 1- and 3-year overall survival rates of 87.7% and 62.4% respectively (36). Zhang et al. demonstrated HIFU’s safety in patients with distance between HCC and main blood vessels (inferior vena cava, main hepatic vein branches and portal vein) of less than 1 cm; indeed, despite this short distance between target and vessels, no vessel’s injury was observed (74). HIFU has been used also in combination with transarterial (TACE). Jin et al. (75) demonstrated a response significantly higher in HIFU + TACE group than in the TACE group alone (44.5%, P<0.05) supported also by survival rates at 1-, 2-, 3- and 5-year that were significantly higher in the HIFU + TACE group (47.2%, 16.7%, 2.8% and 0%, respectively, P<0.01) (75). Moreover, USgFUS ablation is well tolerated in HCC patients with cirrhosis because ultrasound energy travels much better in water than in air therefore the presence of ascites actually facilitates energy propagation to the lesion lowering the chance of adverse reactions (35,76).
The recent introduction of MRgFUS could be another step further in non-invasive treatment of HCC. MR can depict far better the anatomy of liver and indeed it is, actually, considered the reference standard for HCC diagnosis (77,78). Moreover the MR can provide real-time in-procedure quantification of thermal dose and thermal damage to targeted tissue allowing a more precise evaluation of treatment response. Our experience is too limited but our patient was successfully treated without side effects demonstrating safety and efficacy of treatment. Pathological correlation obtained at liver transplant confirmed total ablation of target tissue with a scar detected where the HCC used to be. As for the treatment of pancreatic cancer, the use of general anaesthesia and controlled respiration allow us to overwhelm problems related to breathing movements using a feasible and adequate approach. However the improvement of real-time motion tracking devices will be a major breakthrough in treatment of upper abdominal organs and will lead to further diffusion of this technique (67).
Limitations
Despite these promising results, there are some limitations of technique that need to be addressed.
The upper abdominal organs lay behind the ribcage. Bone reflects and refracts the ultrasound that induces tissue overheating on the bone surface itself (79). Moreover this physical obstacle denies targeting of lesions located behind the ribs. A technique based on the time-reversal process to focus HIFU through the ribcage is under development (80) and will be probably implemented in close future.
The second important limitation of MRgFUS is related to movement of abdominal organs. MR is only capable of near real-time imaging and anatomic monitoring therefore organ movements (up to 20 mm during respiration) may significantly affect lesions targeting and ablation efficacy (81). Our approach including controlled breath-hold allows precise MRgFUS ablations however this is a roughly technique and in the next future will be replaced by more sophisticated methods of targeting like the adaptive method, in which the HIFU beam and the temperature monitoring automatically follow in real-time the motion of the target organ basing on pre-generated models from navigator echoes (67,82).
Conclusions
Our preliminary experience demonstrates that MRgFUS is feasible and safe for the ablation of selected liver and pancreatic tumours. The breathing-control approach was shown to be safe without significant errors in lesion targeting or thermometry. Notwithstanding these results, further developments in real-time motion tracking and thermal mapping techniques are indispensable to consolidate and expand the field of application of this technique in abdominal organs.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Giusi Irma Forte and Giorgio Russo) for the series “High intensity focused ultrasounds” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2014.09.03). The series “High intensity focused ultrasounds” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- American Cancer Society. Cancer Facts & Figures 2013. Atlanta: American Cancer Society; 2013.
- Jemal A, Thomas A, Murray T, et al. Cancer statistics, 2002. CA Cancer J Clin 2002;52:23-47. [PubMed]
- Hariharan D, Saied A, Kocher HM. Analysis of mortality rates for pancreatic cancer across the world. HPB (Oxford) 2008;10:58-62. [PubMed]
- Hidalgo M. Pancreatic cancer. N Engl J Med 2010;362:1605-17. [PubMed]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [PubMed]
- Jarboe J, Saif MW. First line therapy for metastatic pancreatic cancer. JOP 2013;14:340-3. [PubMed]
- Michl P, Gress TM. Current concepts and novel targets in advanced pancreatic cancer. Gut 2013;62:317-26. [PubMed]
- Li D, Abbruzzese JL. New strategies in pancreatic cancer: emerging epidemiologic and therapeutic concepts. Clin Cancer Res 2010;16:4313-8. [PubMed]
- Neureiter D, Jäger T, Ocker M, et al. Epigenetics and pancreatic cancer: pathophysiology and novel treatment aspects. World J Gastroenterol 2014;20:7830-48. [PubMed]
- Devita VT Jr, Hellman S, Rosenberg SA. eds. Cancer: principles and practice of oncology. Philadelphia, Pa: Lippincott Williams & Wilkins, 2001.
- Herreros-Villanueva M, Hijona E, Cosme A, et al. Adjuvant and neoadjuvant treatment in pancreatic cancer. World J Gastroenterol 2012;18:1565-72. [PubMed]
- Kim SC, Kim YH, Park KM, et al. Pancreatic cancer surgery: the state of the art. Curr Drug Targets 2012;13:764-71. [PubMed]
- Wilkowski R, Wolf M, Heinemann V. Primary advanced unresectable pancreatic cancer. Recent Results Cancer Res 2008;177:79-93. [PubMed]
- Ahmed M, Brace CL, Lee FT Jr, et al. Principles of and advances in percutaneous ablation. Radiology 2011;258:351-69. [PubMed]
- Tempero MA, Arnoletti JP, Behrman S, et al. Pancreatic adenocarcinoma. J Natl Compr Canc Netw 2010;8:972-1017. [PubMed]
- Elias D, Baton O, Sideris L, et al. Necrotizing pancreatitis after radiofrequency destruction of pancreatic tumours. Eur J Surg Oncol 2004;30:85-7. [PubMed]
- El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011;365:1118-27. [PubMed]
- Murray CJ, Richards MA, Newton JN, et al. UK health performance: findings of the Global Burden of Disease Study 2010. Lancet 2013;381:997-1020. [PubMed]
- Wang YC, Wei LJ, Liu JT, et al. Comparison of Cancer Incidence between China and the USA. Cancer Biol Med 2012;9:128-32. [PubMed]
- Chen CJ, Yang HI, Su J, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006;295:65-73. [PubMed]
- Akriviadis EA, Llovet JM, Efremidis SC, et al. Hepatocellular carcinoma. Br J Surg 1998;85:1319-31. [PubMed]
- Okuda K, Ohtsuki T, Obata H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer 1985;56:918-28. [PubMed]
- Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg 2000;232:10-24. [PubMed]
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-9. [PubMed]
- Chan AC, Poon RT, Ng KK, et al. Changing paradigm in the management of hepatocellular carcinoma improves the survival benefit of early detection by screening. Ann Surg 2008;247:666-73. [PubMed]
- Hasegawa K, Kokudo N, Makuuchi M, et al. Comparison of resection and ablation for hepatocellular carcinoma: a cohort study based on a Japanese nationwide survey. J Hepatol 2013;58:724-9. [PubMed]
- Lam CM, Ng KK, Poon RT, et al. Impact of radiofrequency ablation on the management of patients with hepatocellular carcinoma in a specialized centre. Br J Surg 2004;91:334-8. [PubMed]
- Toshikuni N, Takuma Y, Goto T, et al. Prognostic factors in hepatitis C patients with a single small hepatocellular carcinoma after radiofrequency ablation. Hepatogastroenterology 2012;59:2361-6. [PubMed]
- Llovet JM, Real MI, Montaña X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002;359:1734-9. [PubMed]
- Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002;35:1164-71. [PubMed]
- Cheung TT, Ng KK, Poon RT, et al. Tolerance of radiofrequency ablation by patients of hepatocellular carcinoma. J Hepatobiliary Pancreat Surg 2009;16:655-60. [PubMed]
- Doffoël M, Bonnetain F, Bouché O, et al. Multicentre randomised phase III trial comparing Tamoxifen alone or with Transarterial Lipiodol Chemoembolisation for unresectable hepatocellular carcinoma in cirrhotic patients (Fédération Francophone de Cancérologie Digestive 9402). Eur J Cancer 2008;44:528-38. [PubMed]
- Orsi F, Zhang L, Arnone P, et al. High-intensity focused ultrasound ablation: effective and safe therapy for solid tumors in difficult locations. AJR Am J Roentgenol 2010;195:W245-52 [PubMed]
- Wu F, Wang ZB, Chen WZ, et al. Advanced hepatocellular carcinoma: treatment with high-intensity focused ultrasound ablation combined with transcatheter arterial embolization. Radiology 2005;235:659-67. [PubMed]
- Wu F. Extracorporeal high intensity focused ultrasound in the treatment of patients with solid malignancy. Minim Invasive Ther Allied Technol 2006;15:26-35. [PubMed]
- Ng KK, Poon RT, Chan SC, et al. High-intensity focused ultrasound for hepatocellular carcinoma: a single-center experience. Ann Surg 2011;253:981-7. [PubMed]
- Schlesinger D, Benedict S, Diederich C, et al. MR-guided focused ultrasound surgery, present and future. Med Phys 2013;40:080901 [PubMed]
- Jenne JW, Preusser T, Günther M. High-intensity focused ultrasound: principles, therapy guidance, simulations and applications. Z Med Phys 2012;22:311-22. [PubMed]
- Orsi F, Arnone P, Chen W, et al. High intensity focused ultrasound ablation: a new therapeutic option for solid tumors. J Cancer Res Ther 2010;6:414-20. [PubMed]
- Cheung TT, Poon RT, Jenkins CR, et al. Survival analysis of high-intensity focused ultrasound therapy vs. transarterial chemoembolization for unresectable hepatocellular carcinomas. Liver Int 2014;34:e136-43. [PubMed]
- Chan AC, Cheung TT, Fan ST, et al. Survival analysis of high-intensity focused ultrasound therapy versus radiofrequency ablation in the treatment of recurrent hepatocellular carcinoma. Ann Surg 2013;257:686-92. [PubMed]
- Muller A, Petrusca L, Auboiroux V, et al. Management of respiratory motion in extracorporeal high-intensity focused ultrasound treatment in upper abdominal organs: current status and perspectives. Cardiovasc Intervent Radiol 2013;36:1464-76. [PubMed]
- Cheung TT, Fan ST, Chan SC, et al. High-intensity focused ultrasound ablation: an effective bridging therapy for hepatocellular carcinoma patients. World J Gastroenterol 2013;19:3083-9. [PubMed]
- Ni S, Liu L, Shu Y. Sequential transcatheter arterial chemoembolization, three-dimensional conformal radiotherapy, and high-intensity focused ultrasound treatment for unresectable hepatocellular carcinoma patients. J Biomed Res 2012;26:260-7. [PubMed]
- Dick EA, Gedroyc WM. ExAblate magnetic resonance-guided focused ultrasound system in multiple body applications. Expert Rev Med Devices 2010;7:589-97. [PubMed]
- Medel R, Monteith SJ, Elias WJ, et al. Magnetic resonance-guided focused ultrasound surgery: Part 2: A review of current and future applications. Neurosurgery 2012;71:755-63. [PubMed]
- Hynynen K, Damianou C, Darkazanli A, et al. The feasibility of using MRI to monitor and guide noninvasive ultrasound surgery. Ultrasound Med Biol 1993;19:91-2. [PubMed]
- Al-Bataineh O, Jenne J, Huber P. Clinical and future applications of high intensity focused ultrasound in cancer. Cancer Treat Rev 2012;38:346-53. [PubMed]
- Anzidei M, Napoli A, Sandolo F, et al. Magnetic Resonance-Guided Focused Ultrasound Ablation in Abdominal Moving Organs: A Feasibility Study in Selected Cases of Pancreatic and Liver Cancer. Cardiovasc Intervent Radiol 2014; [Epub ahead of print]. [PubMed]
- Anzidei M, Marincola BC, Bezzi M, et al. Magnetic Resonance-Guided High-Intensity Focused Ultrasound Treatment of Locally Advanced Pancreatic Adenocarcinoma: Preliminary Experience for Pain Palliation and Local Tumor Control. Invest Radiol 2014; [Epub ahead of print]. [PubMed]
- Jung SE, Cho SH, Jang JH, et al. High-intensity focused ultrasound ablation in hepatic and pancreatic cancer: complications. Abdom Imaging 2011;36:185-95. [PubMed]
- Kennedy JE, Wu F, ter Haar GR, et al. High-intensity focused ultrasound for the treatment of liver tumours. Ultrasonics 2004;42:931-5. [PubMed]
- Illing RO, Kennedy JE, Wu F, et al. The safety and feasibility of extracorporeal high-intensity focused ultrasound (HIFU) for the treatment of liver and kidney tumours in a Western population. Br J Cancer 2005;93:890-5. [PubMed]
- Fite BZ, Liu Y, Kruse DE, et al. Magnetic resonance thermometry at 7T for real-time monitoring and correction of ultrasound induced mild hyperthermia. PLoS One 2012;7:e35509 [PubMed]
- Wu F, Wang ZB, Zhu H, et al. Feasibility of US-guided high-intensity focused ultrasound treatment in patients with advanced pancreatic cancer: initial experience. Radiology 2005;236:1034-40. [PubMed]
- Hwang JH, Wang YN, Warren C, et al. Preclinical in vivo evaluation of an extracorporeal HIFU device for ablation of pancreatic tumors. Ultrasound Med Biol 2009;35:967-75. [PubMed]
- Xie B, Li YY, Jia L, et al. Experimental ablation of the pancreas with high intensity focused ultrasound (HIFU) in a porcine model. Int J Med Sci 2010;8:9-15. [PubMed]
- Khokhlova TD, Hwang JH. HIFU for palliative treatment of pancreatic cancer. J Gastrointest Oncol 2011;2:175-84. [PubMed]
- He SX, Wang GM. The noninvasive treatment of 251 cases of advanced pancreatic cancer with focused ultrasound surgery. 2nd International Symposium on Therapeutic Ultrasound. Seattle, Washington, USA 2002:51-6.
- Xu YQ, Wang GM, Gu YZ, et al. The acesodyne effect of high intensity focused ultrasound on the treatment of advanced pancreatic carcinoma. Clin Med J China 2003;10:322-3.
- Wang X, Sun JZ. Preliminary study of high intensity focused ultrasound in treating patients with advanced pancreatic carcinoma. Chin J Gen Surg 2002;17:654-5.
- Xiong LL, Hwang JH, Huang XB, et al. Early clinical experience using high intensity focused ultrasound for palliation of inoperable pancreatic cancer. JOP 2009;10:123-9. [PubMed]
- Xie DR, Chen D, Teng H. A multicenter non-randomized clinical study of high intensity focused ultrasound in treating patients with local advanced pancreatic carcinoma. Chin J Clin Oncol 2003;30:630-4.
- Wang K, Chen Z, Meng Z, et al. Analgesic effect of high intensity focused ultrasound therapy for unresectable pancreatic cancer. Int J Hyperthermia 2011;27:101-7. [PubMed]
- Zhao H, Yang G, Wang D, et al. Concurrent gemcitabine and high-intensity focused ultrasound therapy in patients with locally advanced pancreatic cancer. Anticancer Drugs 2010;21:447-52. [PubMed]
- Holbrook AB, Santos JM, Kaye E, et al. Real-time MR thermometry for monitoring HIFU ablations of the liver. Magn Reson Med 2010;63:365-73. [PubMed]
- Ries M, de Senneville BD, Roujol S, et al. Real-time 3D target tracking in MRI guided focused ultrasound ablations in moving tissues. Magn Reson Med 2010;64:1704-12. [PubMed]
- Weeks EM, Platt MW, Gedroyc W. MRI-guided focused ultrasound (MRgFUS) to treat facet joint osteoarthritis low back pain--case series of an innovative new technique. Eur Radiol 2012;22:2822-35. [PubMed]
- Napoli A, Anzidei M, Marincola BC, et al. Primary pain palliation and local tumor control in bone metastases treated with magnetic resonance-guided focused ultrasound. Invest Radiol 2013;48:351-8. [PubMed]
- Napoli A, Mastantuono M, Cavallo Marincola B, et al. Osteoid osteoma: MR-guided focused ultrasound for entirely noninvasive treatment. Radiology 2013;267:514-21. [PubMed]
- Jang HJ, Lee JY, Lee DH, et al. Current and Future Clinical Applications of High-Intensity Focused Ultrasound (HIFU) for Pancreatic Cancer. Gut Liver 2010;4:S57-61. [PubMed]
- Crocetti L, Della Pina C, Cioni D, et al. Peri-intraprocedural imaging: US, CT, and MRI. Abdom Imaging 2011;36:648-60. [PubMed]
- Xu G, Luo G, He L, et al. Follow-up of high-intensity focused ultrasound treatment for patients with hepatocellular carcinoma. Ultrasound Med Biol 2011;37:1993-9. [PubMed]
- Zhang L, Zhu H, Jin C, et al. High-intensity focused ultrasound (HIFU): effective and safe therapy for hepatocellular carcinoma adjacent to major hepatic veins. Eur Radiol 2009;19:437-45. [PubMed]
- Jin C, Zhu H, Wang Z, et al. High-intensity focused ultrasound combined with transarterial chemoembolization for unresectable hepatocellular carcinoma: long-term follow-up and clinical analysis. Eur J Radiol 2011;80:662-9. [PubMed]
- Cheung TT, Chu FS, Jenkins CR, et al. Tolerance of high-intensity focused ultrasound ablation in patients with hepatocellular carcinoma. World J Surg 2012;36:2420-7. [PubMed]
- Murakami T, Tsurusaki M. Hypervascular benign and malignant liver tumors that require differentiation from hepatocellular carcinoma: key points of imaging diagnosis. Liver Cancer 2014;3:85-96. [PubMed]
- Flores A, Marrero JA. Emerging trends in hepatocellular carcinoma: focus on diagnosis and therapeutics. Clin Med Insights Oncol 2014;8:71-6. [PubMed]
- Kennedy JE, Clarke RL. The effect of the absorbers such as ribs in the HIFU Beam-path on the focal profile. 2nd International Symposium on Therapeutic Ultrasound. Seattle, Washington, USA 2002:185-92.
- Aubry JF, Pernot M, Marquet F, et al. Transcostal high-intensity-focused ultrasound: ex vivo adaptive focusing feasibility study. Phys Med Biol 2008;53:2937-51. [PubMed]
- Davies SC, Hill AL, Holmes RB, et al. Ultrasound quantitation of respiratory organ motion in the upper abdomen. Br J Radiol 1994;67:1096-102. [PubMed]
- Vigen KK, Daniel BL, Pauly JM, et al. Triggered, navigated, multi-baseline method for proton resonance frequency temperature mapping with respiratory motion. Magn Reson Med 2003;50:1003-10. [PubMed]


