
Review of ultrasound mediated drug delivery for cancer treatment: updates from pre-clinical studies
Introduction
Since Persian Queen Atossa’s breast tumor removal by Demokedes (1,2), surgery has been the only option to fight cancer for two millenaries. Patient survival remained low until the development of chemotherapy drugs that attack rapidly dividing cancer cells (1,3,4). However, efficient drug delivery that also minimizes toxicity to healthy cells remains hampered by the difficult penetration of the drug in the vicinity of the cells that cause the disease. In tumors, the transport of drugs indeed encounters several physical barriers and the penetration of therapeutic molecules is often poor and heterogeneous. The barriers to the natural diffusion and convection of drug molecules from the blood vasculature to the surrounding tissue in tumors are mostly consequences of the characteristics of the angiogenic vasculature [for reviews, see (5-8)]. Angiogenesis is a natural phenomenon that arises during development and wound healing. In these natural phenomena, angiogenesis is tightly regulated (9,10). It can also occur during abnormal processes such as tumor growth. In order to sustain its growth, the tumor needs more nutrients and triggers the formation of new vessels.
But since this angiogenesis occurs in an uncontrolled way in tumors, the newly formed vascular network is often abnormal. At the macroscopic level, the vasculature is tortuous, highly branched and chaotic, with dead ends or loops that impair blood flow (11). Its distribution is spatially heterogeneous, resulting in the coexistence of high and low blood vessel density areas. Angiogenesis is thus a highly inefficient process since the presence of these hypo perfused areas produces hypoxia amongst the tumor cells. Due to the lack of perfusion, drugs are not efficiently delivered to the hypoxic tumor cells; moreover, hypoxic cells are also more resistant to radiotherapy, more likely to develop resistances against chemotherapies and more likely to become invasive (12).
If the macroscopic organization of the angiogenic network is disrupted, the structure of the blood vessel is also abnormal on a microscopic level: the adhesions between endothelial cells are weakened, and the perivascular cells tend to detach from the basement membrane around the vessel (13-16). All these phenomena result in the leakage of vessels. Plasma proteins can easily enter into the surrounding tissue, increasing the interstitial fluid pressure (IFP) in the surrounding tissues and thus decreasing the pressure gradient between the inside and the outside of the vessel. As a consequence, the convection of fluid that normally moves the drugs molecules from the blood vessels to the surrounding tissue is hindered (17).
The absence of transvascular flow in some areas of the tumor jeopardizes the homogeneous delivery of drugs. New techniques to improve the delivery of drugs within tumors are needed. In this review, strategies based on mechanical and/or thermal effects of non-invasive and non-destructive ultrasound will be described. As will be discussed, versatile ultrasound beams can indeed interact with the cell membranes, the vessel walls, drug carriers and/or the drug itself.
Ultrasonic drug release at targeted sites
Drug-delivery with ultrasound relies on the interaction between a biocompatible carrier and an acoustic wave. The spatial specificity of the release is established by focusing the waves in the zone to be treated using physical principles and technologies developed in the past for diagnostic and therapeutic ultrasound [such as high intensity focused ultrasound (HIFU) or lithotripsy]. The main challenge in ultrasound-triggered therapy is the design of carriers that are both responsive to ultrasound and biologically active. These agents should be able to carry large payloads and have access, or even accumulate preferentially, within the tumor. These challenges have been addressed by early researchers, such as Tacker and Anderson (18), along with wide and recent international collaborations such as Sonodrugs (19-25). In this section, we will first highlight the mechanisms by which ultrasound can release a payload and then describe various drugs, agents or nucleic acids that have been released with ultrasound in pre-clinical studies.
Drug-delivery mechanisms
The field of ultrasound-enhanced drug-delivery has been strongly influenced by the development of microbubbles (MBs) as contrast agents (26) and liposomes as general drug-delivery carriers (27). The mechanisms underlying drug release via ultrasound can be divided into thermal and mechanical processes, and often a combination of both.
Thermal release
Thermal release involves an ultrasound-induced temperature increase in the treated zone, which results from the absorption of acoustic energy at a rate beyond that of diffusion. This usually implies moderate intensities (several W/cm2), high duty cycles (up to 100%), moderate pressures (100’s of kPa to MPa) and long treatment times (several seconds to 30 minutes) with dedicated focused ultrasound (FUS) transducers. To reduce the required acoustic intensity and limit unspecific heating damage, while guaranteeing its specificity, carriers are often designed to deliver their payload at temperatures just a few degrees above physiological temperature (42-43 °C).
The most common thermally responsive carriers described in the literature are temperature-sensitive liposomes (TSL) (28,29). Liposomes are composed of an aqueous solution inside single or concentric lipid bilayers (30,31). Drugs or agents can be contained within the inner phase (Figure 1A). For example, Doxil, an FDA-approved agent, carries doxorubicin (DOX) while reducing the toxicity of the chemotherapeutic agent (32). The liquid-crystalline phase transition of these liposomes can be selected by modifying the content of their lipid shell. TSLs can also be produced by adding leucine-zipper to the membrane of the liposomes (33). As shown in in-vitro studies, these agents can release up to 80% of their content after 15 minutes of hyperthermia at 43 °C (22). Such TSLs are already well-established because of previous use with other heat sources such as radiofrequency (RF) devices. The primary drawback to both RF and ultrasound hyperthermia remain long treatment times.
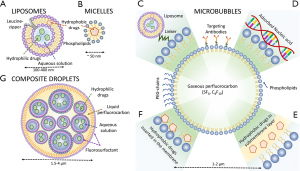
Mechanical release
Drug-delivery can also be performed by inducing high mechanical stresses on drug carriers using short ultrasound pulses. Ultrasound drug-delivery through non-thermal processes requires the presence of micelles (Figure 1B), MBs (Figure 1C-F) or liquid perfluorocarbon droplets (Figure 1G). MBs are used in the clinic for diagnostic ultrasound because of their high echogenicity, their nonlinear scattering and propensity to disrupt under sufficient acoustic pressures (34). Because of their high compressibility, the radius of MBs can vary by a factor of two, leading to important mechanical effects in the surrounding environment (35) that can modify the shell of the microbubble itself. For instance, their oscillations during an ultrasound cycle can cause shedding of its shell. The motion of the shell can move and propel content that was adsorbed on its surface (36). The disruption of the bubble wall can also lead to the release of agents that were bound within the shell or at the core of the bubble. Moreover, the motion of the surrounding fluid induced by the large oscillations of the bubbles facilitates further convection and penetration of the drug. These effects do not depend on temperature, but rather on the peak acoustic pressure and the frequency. Short pulses of a few MPa with total intensities within diagnostic limits (a few hundred mW/cm2 or less) are sufficient, compared to several mW/cm2 of continuous sonication for temperature-induced release. Therefore, microbubble based drug-delivery can be achieved with low-intensity ultrasound systems, often unfocused, or even diagnostic scanners.
These physical phenomena have led to the development and investigation of various types of MBs for drug-delivery. For example, FDA-approved Doxil can be attached on the surface of MBs with the help of molecular linkers, allowing the release of the liposomes during the ultrasound-induced oscillations of the contrast agent (37,38) (Figure 1C). Drugs or DNA can also be adsorbed on the charged surface of the shell (Figure 1D). Moreover, hydrophobic drugs can be inserted below (Figure 1E) or into (Figure 1F) the membrane of the MBs (39) or even added to a polymeric shell (40). The primary drawback of these methods is that most of the content of MBs is gas, rather than drug or gene payload. Moreover, the size of MBs resonating at diagnostic frequencies (3-15 MHz) is close to 1 micrometer, which prevents certain tumor-specific accumulation. To alleviate these issues, liposomes containing gas bubbles have been proposed (41). Polymeric micelles (42), a hydrophobic carrier similar in size to liposomes, were also shown to release their content through stable cavitation (42).
Spontaneous cavitation can be induced in tissue with sufficient peak-negative pressure (tens of MPa). As demonstrated by its effect on kidney stones and tissue, cavitation can disrupt surrounding interfaces, including liposomes or micelles. For instance, short pulses with large pressure were used to deliver the content of liposomes in Somaglino et al. (43). Additionally, mechanical stresses exploited for drug-delivery can also be generated through radiation pressure (44).
The combination of thermal and mechanical stresses can be used to induce the vaporization of gas-precursors and perform drug-delivery without microbubble injection. This often requires the use of perfluorocarbon (PFC) in various forms, from decafluorobutane (45) and perfluoropentane (46) to perfluorohexane (47). These PFC can be confined as liquids within micro or nano droplets thanks to Laplace pressure. When insonified, liquid rapidly converts into gas, leading to disruption of the droplets and rapid expansion of their content. The mechanism of acoustic droplet vaporization was recently explained by Shpak et al. as a form of superfocusing of the acoustic wave within the agent (48). This phenomenon can be triggered by pressures compatible with a diagnostic scanner [3 MPa at 5.5 MHz for few cycle pulses in (49) and 3.5 MPa at 8 MHz for few cycle pulses in (50)], especially with droplets made of PFC, with low-boiling point (49,50).
This concept was used to create nanodroplets (hundreds of nm) that are also effective as ultrasound contrast agents (51). For drug-delivery, they can be mixed in various ways with payload. A nanodroplet of liquid PFC can be added to the content of a liposome to trigger the disruption of the carrier under high pressure ultrasound exposure (52). Other agents can be designed by creating a nanoemulsion of PFC and a hydrophilic (53,54) or hydrophobic solution (46) containing the payload, itself encapsulated in a larger droplet (Figure 1F). The vaporization of the PFC by low-intensity pulses releases the nanoemulsion. This construct allows larger payloads since up to ⅔ of the volume of the droplets can be used to contain drugs. One potential application for these droplets is tissue tattooing for surgical guidance (50).
Liposomes, micelles, MBs and liquid-PFC particles not only need to react specifically to ultrasound, but they should also deliver sufficient payload at the appropriate site. MBs are intravascular agents and do not extravasate. Their circulation lasts several minutes after intravenous bolus injection. Sonication must be performed during the passage of the agents within the target. Longer retention within a tumor can be attained by targeting MBs with tumor-specific antibodies attached on their surface (55). Also, magnetic (56) and radiation forces (57) can be used to slow their progress through the tumor and increase drug-delivery. Because of their size (about 4 microns in diameter), composite droplets behave similarly to MBs and circulate within a restricted amount of time (58). Liposomes and micelles, on the other hand, can be produced to be retained specifically in cancer cells (59,60). Indeed, when their size is below 200 nm, the porous vascular structure of angiogenic tumors allows their passage to the extravascular space. These agents are thus injected several hours, or days, before sonication to allow preferential accumulation in the tumor.
Drugs and targets
Several groups have used these carriers (liposomes, micelles, MBs, liquid droplets) and mechanisms (thermal, mechanical, vaporization) to perform in vivo drug-delivery. Only a fraction of several hundred articles on the subject can be discussed in this review. After the discovery of thermosensitive liposomes, Tacker and Anderson (18) rapidly performed ultrasound experiments on tumor-bearing mice. By heating the tumor with ultrasound beyond the 42 °C transition of their methotraxate-filled liposomes, they showed an elevated accumulation of the chemotherapeutic drug in the tumor in this bladder-cancer model. The same group demonstrated the encapsulation of cisplatin (61). More recent studies on ultrasound-released liposomal cisplatin showed cancer regression in mice (29,62).
Paclitaxel was also inserted in ultrasound-sensitive agents. For instance, Rapoport et al. (63) observed the regression of pancreatic-tumors in mice after administration of an ultrasound-sensitive PFC nanoemulsion.
DOX, a cancer chemotherapeutic, was encapsulated in ultrasound-sensitive carriers by several groups (64-66). In 1994, Ning et al. (67) showed a 10-fold increase in the release of DOX using stealth liposomes. More recently, temperature-sensitive liposomal DOX were tested in the context of magnetic resonance guided HIFU (MRgHIFU) highlighting in vivo increased accumulation of the drug within tumor models in rabbits (68). In another study, the specific release of DOX from liposomes during ultrasound treatment lead to complete regression of tumors in mice (69). DOX was included within liposomes attached to the surface of MBs (70), and lead to a reduction of tumor growth in rats (71). DOX was also confined within micelles (72) and polymeric nanoparticles, but Cochran et al. (73) obtained better encapsulation of the hydrophobic molecule paclitaxel in this later construct.
Thirty-two years after the first ultrasound drug-delivery in animals, clinical trials are approved to begin in Oxford to release DOX from liposomes with the help of a HIFU system for the treatment of liver metastasis (74).
In parallel with the delivery of conventional chemotherapeutic agents, other paths are being explored where the advantages of MBs and ultrasound are more specifically exploited. For instance, MBs and liposomes, in conjunction with ultrasound, can deliver nucleic acids for gene therapy (75,76). Indeed, not only can ultrasound-sensitive carriers enable the passage of nucleic acids in specific zones (77), but they can also protect this genetic material from enzymatic activity in the blood. Initially proposed with liposomes (78), ultrasound gene delivery was rapidly performed with cationic lipid transfection complex showing a 270-fold increase in DNA expression (79). Negishi et al. (80) achieved gene silencing effects by introducing small interfering RNA (siRNA) within cells using bubbles, liposomes and ultrasound. Anti-cancerous effects were obtained with EGFR-directed siRNA or thymidine kinase and ganciclovir in murine carcinoma, hence reducing tumor growth (81,82). Plasmid which can trigger the expression of reporter genes were also targeted to tumor cells in mice using cationic bubbles (83).
Interestingly, ultrasound-mediated delivery was also performed with oncolytic viruses. These viruses can kill and transfect tumor cells, but can also self-replicate. However, their passage into the extravascular space remains limited. The use of MBs and ultrasound can increase the expression of these viruses by a factor of 50 (84).
In the case of infiltrating or invading cancers, the localized approach described before might be insufficient, even with an efficient drug-delivery device. To encourage the immune system to identify and destroy remote tumor cells, several approaches of cancer vaccination were suggested with ultrasound (85). Not only can HIFU inherently cause the immune system to recognize tumor cells (86,87), but delivery of mRNA, plasmid DNA and cancer antigens can also be promoted via ultrasound-sensitive agents (88). For instance, by introducing plasmid DNA to antigen presenting cells using MBs, lipoplexes and ultrasound, Un et al. (89) demonstrated encouraging antitumor effect and improved survival rate.
Despite these advances, ultrasound-induced drug delivery is not a “magic bullet”, mainly because most of the injected drugs still accumulate in non-cancerous tissue. Recently, Bezagu et al. (90) proposed to produce the drugs in-situ by inducing a chemical reaction through ultrasound release of composite droplets. By exploiting the strong hydrophobicity and lipophobicity of the PFC composing the droplets, two prodrugs can be isolated from each other until ultrasound-induced release, which becomes a condition for the very existence of the active drug. Such a concept guarantees that any pharmaceutical effect will be isolated within the specific zone of the ultrasound-induced delivery.
Ultrasound-enhanced permeability of biological barriers
Once the payload has been delivered intravenously, the permeability of the vascular walls is critical to deliver the drug to pathological tissues.
The particular case of the brain: blood-brain barrier (BBB) disruption
Specific organs such as brain and retina present much more impermeable vasculature which acts as a barrier blocking almost all therapeutic access. The main example is the BBB which consists of tight junctions between endothelial cells in the brain (91). This barrier is a complex biological system involving a large number of specific proteins and receptors and is therefore difficult for large and hydrophilic molecules to cross (92,93). In its normal state, it protects the brain from infections as well as ensures its homeostasis (92).
Ultrasound combined with the injection of MBs has been shown to safely and reversibly disrupt the BBB in a multitude of studies since 2001 (94). Since then, as recently reviewed by Aryal et al. (95), a large number of animal studies have been conducted to determine the optimal acoustic parameters and timings and the optimal properties for MBs. Studies have also measured the obtained enhanced permeability in an effort to understand the mechanisms of interaction of sonicated MBs with endothelial cells and the induced bio-effects, and to assess the potential tissue damage and its time course for this reversible process (Figure 2). Under proper conditions, it was possible to reach locally and reversibly the same vascular permeability as the one measured in peripheral organs without any adverse effects for several hours (97). It is thus a very promising tool for targeted drug delivery.
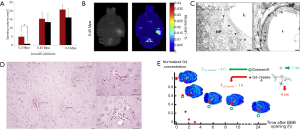
Regarding cancer treatment, ultrasound was shown to enhance delivery of several chemotherapy agents across the BBB in healthy brain, including: Herceptin (98), liposomal DOX (99), methotrexate (100), cytarabine (101), and DOX (102). Gene delivery and transfection was also demonstrated in normal brain and ultrasound-aided gene therapy was achieved for cancer therapy (103-106).
However, it is well documented that neoangiogenic vascular networks present vessels with already altered BBB (the so-called blood-tumor barrier or BTB) as well as longer residence times for drugs. Therefore, the potential benefit of ultrasound was not obvious. Nevertheless, it was recently shown that tumor vascular endothelium becomes more permeable after ultrasound. As a result, since 2010, many preclinical studies have evaluated the therapeutic gain of using ultrasonic BTB disruption for brain tumor treatments. Liu et al. recently reviewed the current status of drug delivery to brain tumors using ultrasound (107). Table 1 summarizes the current status of the animal cancer models that were used and the anti-tumor drugs that were injected as well as the main acoustic parameters used. As illustrated in Figure 3, these studies demonstrated that ultrasound can enhance delivery of a wide variety of drugs to tumors [for example, +200% BCNU delivery to C6 glioma bearing rats (119)]. Most studies showed significant control of tumor growth or even full regression (Figure 3B). This indicates that therapeutic levels of drugs were delivered in targeted regions and that drugs sufficiently diffused inside tumors. When measured, the median survival time was improved as well (Figure 3C). In addition, two interesting concepts were successfully tested. First, encapsulating the drug payload into magnetic nanoparticles, liposomes, or MBs enables less peripheral toxicity. This method limits the required dose, increases drug lifetime in the circulation and achieves high local concentration after drug release (113,117,119,122,123). Second, biologically targeting theses drug cargos by grafting specific proteins on their surfaces enables further increase of the local concentration before sonication (113,114,124).
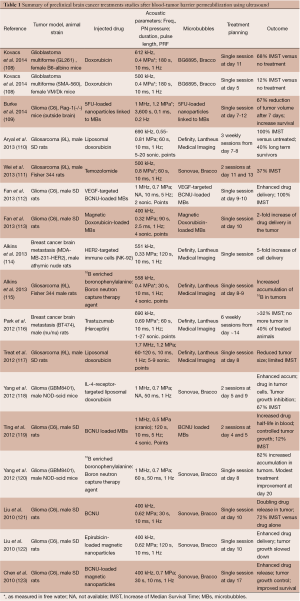
Full table
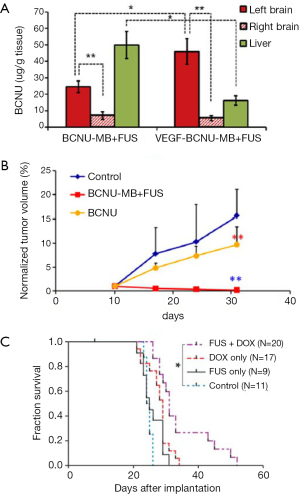
Although qualitatively consistent with each other and very encouraging, these studies present significant discrepancies in their quantitative outcomes (tumor growth, mean survival gain). Indeed, they are difficult to compare since they were conducted under very different experimental conditions. Namely the acoustic frequency ranged from 400 kHz to 1.7 MHz and the size of the therapeutic agents that have been tested ranges from less than 1 nm molecules (200 Da) to cells of several microns in size. The spatial extent of BTB disruption, depending on acoustic frequency, peak negative pressure, geometry of the transducer and number of sonication points, will affect the efficiency of drug delivery. The number of treatment sessions and their timings are also important to consider (110). The influence of key parameters such as anesthetics used during BBB disruption should also be considered (125).
To date, all brain tumor models were orthotopic murine models which are much less infiltrating than human gliomas. Data obtained on tumor models that better mimic human pathology are needed to ensure translation of these promising results. In order to extrapolate to humans, safety data is critical. Although several histological studies have been conducted in healthy brain after BBB disruption with a wide number of acoustic parameters, only few papers have studied tissue damage and repair in tumors. In particular, since tumor vasculature might be easier to disrupt, it would be of great importance to determine whether or not hemorrhage results at lower acoustic thresholds than in the rest of brain parenchyma. The propagation of hemorrhage through blood leakage could jeopardize the advantages of this technique. The safest parameters showing no occurrence of hemorrhage should be identified.
Enhanced permeability of cell membranes: sonoporation
A large number of in vitro studies have demonstrated that ultrasound alone or combined with MBs can efficiently increase cell membrane permeability in cell cultures (126). This technique has been used intensively for the last 20 years as a competitor to electroporation to deliver chemical substances, a majority of them being DNA plasmids. However, like for BBB disruption, quite a wide range of acoustic conditions were proposed. Moreover, the mechanisms of interaction of ultrasound, MBs and cell membranes and their bio-effects are not fully elucidated as recently reviewed by Mullin et al. for nanoparticle delivery (127). In a recent paper, Lentacker et al. categorized the experiments that have been published to date according to the probable mechanisms they exploit (128): bio-effects induced (I) by stable cavitation of MBs; (II) by inertial cavitation of MBs; or (III) by ultrasound without MBs. It is hypothesized that in the latter case, ultrasound-induced cavitation of tissue dissolved gases or acoustic microstreaming or shear stresses could be responsible for the observed bio-effects. Studies have also reported different bio-effects responsible for drug uptake in cells: direct pore formation in the membrane or activation of repair mechanisms aiming at replacing portions of the phospholipid membrane and thus stimulating endocytosis through different biological signaling pathways.
In vivo gene delivery and transfection for cancer treatment
This review paper focuses on in vivo preclinical studies related to cancer. Around half of the currently published papers in this area concern non-viral gene delivery and gene transfection. Early work included proofs of concept for increased gene delivery in tumors using ultrasound alone, but under very strong acoustic conditions i.e., for example using shock waves (129-135). As an example, Anwer et al. (132) obtained without MBs a major increase of IL-12 gene transfer (up to 270-fold increase) limited to tumor endothelial cells. It was sufficient to inhibit tumor growth in mice. Hayashi et al. showed that drug-encapsulating liposomes could enhance gene transfection and chemotherapy after sonication better than independent MBs and drug injections (134).
Most of the recent in vivo studies used MBs and more modest acoustic conditions (i.e., lower mechanical index and lower duty cycle) while showing higher gene expressions (136-146). Sakakima used IFN-γ plasmid cDNA to treat human hepatic cancer (SK-Hep1) in mice with a reduction of tumor size (137). They injected the MBs mixed together with the plasmid directly in the tumor right before sonication. Using intravenous injections and a standard diagnostic ultrasound scanner, Hauff et al. increased tumor doubling time in capan-1 tumor mice treated by p16 tumor suppressor gene for 5 weekly sessions (138). The originality of their approach is the encapsulation of the plasmid DNA into the ultrasound contrast agent. Li et al. and Tsai et al. optimized the ultrasound parameters to enable prolonged gene expression in muscles and tumors (139,140). For instance, Li et al. found the best transfection efficiency for an acoustic frequency of 1 MHz, an intensity of 4 W/cm2 and a duty cycle of 25%. More recent work demonstrated promising treatment outcomes for various genes in different murine models (141,142,144). Liao et al. (144) combined anti-angiogenic gene therapy with either chemotherapy or immunotherapy. Interestingly, they performed ultrasound aided gene transfection in muscles distant from the tumors and observed an additional therapeutic effect due to gene therapy.
Rychak and Klibanov recently published a review paper on DNA delivery using MBs and ultrasound (147). Although it would be the easiest protocol to implement in clinics, very few studies have injected the MBs into the blood stream so far (138,143). The therapeutic gain reported in these studies is likely to be due to an enhanced vascular permeability rather than to cancer cell sonoporation. Indeed, due to their size, MBs are likely to stay in the vasculature. To a smaller extent than in the brain, the permeability of vessel walls in peripheral organs can be increased as well. When genes and MBs are directly injected into the tumors, it is not clear how far they can diffuse from injection points to reach a maximum of cancer cells. Obtaining efficient gene transfection at a distance of blood vessels after systemic injection would be critical to the clinical translation of the technique.
Chemotherapies
Twenty years ago, it was observed in rodents and patients that shock waves were potentiating concomitant chemotherapy, likely due to enhanced permeability of biological membranes (148,149). In 2007, Iwanaga showed massive tumor regression in Ca9-22 tumor bearing mice after sonoporation and treatment by either bleomycin or a toxin-expressing plasmid (150). Similarly, Matsuo et al. demonstrated increased tumor regression after sonoporation of melanoma treated with melphalan (151). In these studies, MBs and therapeutic agents were both directly injected into the tumor thus the protocol remained invasive.
Recently, Yamatomo et al. demonstrated that boron neutron capture therapy of squamous cell carcinoma benefited from sonoporation of the tumor, with the drug injected intraperitoneally (152). Sato et al. compared intravenous and intralymphatic administration of MBs and cisplatin to treat lymph node metastasis (153). They only found significant improvements when using intralymphatic injections. Kotopoulis et al. showed an enhanced effect of gemcitabine after sonoporation on a mouse model of human pancreatic adenocarcinoma (154). The same authors recently published the first clinical results of sonoporation enhanced drug delivery in pancreatic cancer patients (155). The co-injection of Sonovue® MBs in the blood stream with gemcitabine followed by sonication with a clinical ultrasound scanner resulted in a slowdown of the tumor growth and a significant extension of the healthy period of life of these patients bearing a very aggressive cancer. The question remains whether cancer cells really experienced sonoporation or whether therapeutic gain came from a MB-induced increase of vascular permeability and thus enhanced gemcitabine delivery.
Several other molecules have been successfully tested in vitro but not yet in vivo.
Combined sonoporation and local drug release
Interestingly, Yudina et al. combined cavitation-induced sonoporation and thermally induced drug release from thermosensitive liposomes. In tumor bearing mice, they achieved significant cellular internalization of TO-PRO-3, a cell impermeable molecule after intravenous injection of MBs and drug-loaded thermosensitive liposomes (156).
Ultrasonic activation of drugs (sonodynamics)
The needs to enhance the effectiveness and reduce the toxicity of chemotherapy are major drivers for development of new drug delivery techniques. To this end, photodynamic therapy (PDT) and more recently sonodynamic therapy (SDT) have been shown to enable precise destruction of tumor cells without damaging adjacent normal cells.
Certain drugs, including chemical agents such as hematoporphyrin (HDT) and 5-aminolevulinic acid (5-ALA), are known to preferentially accumulate in tumors cells. On their own, these agents are inert and non-toxic. However, the agents can be activated by light of a certain wavelength, in a technique known as PDT, to induce apoptosis of the tumor cells. The process of PDT generates oxygen free radicals that result in DNA damage and ultimately apoptosis (157). PDT is used clinically to treat various cancers although it has inherent qualities that could limit its clinical utility (158,159). Light cannot penetrate deep within tissue and therefore it can only reach superficial tumors and still be non-invasive. If deep tumors are the target, the procedure will require inserting a fiberoptic probe into the tissue, raising the risk of potential infection. Furthermore, the light may diffuse irregularly throughout the tumor and result in an incomplete treatment (160,161).
With recent advancements in the field of SDT, the technique has shown potential to overcome the limitations of PDT (96). SDT employs ultrasound rather than light to activate many of the same chemical agents. Focused ultrasound energy has been observed to excite agents including HDT, Rose Bengal or 5-ALA, after accumulation in tumor cells, to induce apoptosis of the targeted cells (162,163). Although the mechanism is not widely understood, it may be similar to that for PDT including the involvement of reactive oxygen species to lead to apoptosis. Alternatively, the shear forces generated by FUS of appropriate parameters could potentially induce damage sufficient to trigger cell death (164-167). FUS of low to moderate intensity (e.g., 1.0 MHz, 10 to 25 W/cm2), and applied continuously for 5 min, has been shown to be effective in a rat intracranial tumor model at inducing apoptosis and reducing the overall tumor size (162,163).
As compared to PDT, SDT could provide a true noninvasive option even for deep seated tumors. Ultrasound energy does not have limited depth penetration or irregular diffusion within the tumor tissue, issues common for light. Therefore, FUS could provide a more conformal treatment of the tumor, via homogeneous delivery of energy and apoptosis throughout the entire tumor. FUS is also more highly focused than light, thus minimizing potential damage or toxicity to intervening or adjacent tissue (168,169).
The field of SDT is still early stage, and clinical utility has yet to be realized. Early research suggests low-power ultrasound to induce non-thermal effects is most effective. While further research must be conducted on the mechanisms responsible for this phenomenon, and the development and optimization of sonosensitizers and ultrasound parameters, SDT holds promise in non-invasive cancer treatment (169,170).
Potential role of combination therapy
The standard practice for treatment of many cancers today involves multiple different treatment modalities, such as the combination of surgery, radiation and chemotherapy. The development of a technique such as FUS to enable more efficient and localized drug delivery could reduce the need for standard systemic chemotherapy. However, optimal treatment for some cancers may still require a combination therapy approach.
There are many different combinations that could be envisioned that will require further investigation before clinical practice is altered. One such combination therapy could include FUS hyperthermia of a tumor followed by FUS-enhanced delivery of chemotherapy drugs to the tumor bed and a margin of adjacent tissue (171,172). The hyperthermia would aim for bulk necrosis of the tumor whereas the localized chemotherapy could protect against any tumor cells not identified and targeted via hyperthermia. Similarly, traditional surgical removal of the tumor could be followed by FUS-enhanced chemotherapy.
Therapies to combine FUS-enhanced drug delivery with radiation therapy may also prove beneficial. These therapies combined with FUS ablation could also prove synergistic. There has been research indicating that hyperthermia can make tumors more sensitive and receptive to radiation (173). FUS-enhanced drug delivery methods often employ the hyperthermia capabilities of FUS. In this case, hyperthermia can stimulate blood flow to the tumor, increasing its oxygenation, enhancing its metabolic rate and increasing the effectiveness of radiation therapy. This is particularly useful in the case of hypoxic tumors that are ordinarily difficult to treat via radiation. This sensitization via FUS can enable treatment using lower doses of radiation and thus more minimal side effects (174,175). Furthermore, FUS ablation could be added to this treatment regimen.
The ability of FUS in combination with ultrasound contrast agents (MBs) to temporarily and reversibly open the BBB is a promising technique enabling more effective delivery of drugs to the brain. Oftentimes, getting through the BBB is only one issue, and the tumor barrier itself may prevent effective delivery of therapies. Therefore, it could be useful to combine FUS-enhanced BBB opening with FUS-enhanced drug delivery at the site of the tumor via drug-loaded liposomes (e.g., Thermodox) or nanoparticles (176). These carrier vehicles, designed to respond to a specific threshold of heat or pressure produced by FUS, could then release drugs locally at the tumor for more effective uptake. Furthermore, FUS-induced sonoporation at the brain/tumor barrier could enhance permeation of drugs into the tumor.
Conclusions
Ultrasonic drug delivery has been limited to in vitro experiments for decades. Promising in vivo results have accumulated in the past ten years and this field is now nearing clinical trials. Ultimately, FUS-enhanced drug delivery is one tool in the armamentarium for optimal treatment of cancer. It may be enough on its own in some cases, but in other more complex cases, a combination therapy approach may be more effective.
Acknowledgments
The authors thank Benjamin Sela for editing the references.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Giusi Irma Forte and Giorgio Russo) for the series “High intensity focused ultrasounds” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: J-F Aubry is consultant for the Focused Ultrasound Foundation, Charlottesville, VA. Neal Kassell is the founder and chairman of the Focused Ultrasound Foundation, as well as a shareholder of InSightec Ltd.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mukherjee S. eds. The emperor of all maladies: a biography of cancer. New York: Simon and Schuster; 2011.
- Herodotus. The Histories 440BC:3.133. Available online: http://classics.mit.edu/Herodotus/history.3.iii.html
- DeVita VT Jr, Chu E. A history of cancer chemotherapy. Cancer Res 2008;68:8643-53. [PubMed]
- Chabner BA, Roberts TG Jr. Timeline: Chemotherapy and the war on cancer. Nat Rev Cancer 2005;5:65-72. [PubMed]
- Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer 2006;6:583-92. [PubMed]
- Fukumura D, Jain RK. Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization. Microvasc Res 2007;74:72-84. [PubMed]
- Frenkel V. Ultrasound mediated delivery of drugs and genes to solid tumors. Adv Drug Deliv Rev 2008;60:1193-208. [PubMed]
- Goel S, Duda DG, Xu L, et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev 2011;91:1071-121. [PubMed]
- Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 1986;315:1650-9. [PubMed]
- Jain RK. Molecular regulation of vessel maturation. Nat Med 2003;9:685-93. [PubMed]
- Jain RK. Determinants of tumor blood flow: a review. Cancer Res 1988;48:2641-58. [PubMed]
- Pennacchietti S, Michieli P, Galluzzo M, et al. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell 2003;3:347-61. [PubMed]
- Chang YS, di Tomaso E, McDonald DM, et al. Mosaic blood vessels in tumors: frequency of cancer cells in contact with flowing blood. Proc Natl Acad Sci U S A 2000;97:14608-13. [PubMed]
- Morikawa S, Baluk P, Kaidoh T, et al. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol 2002;160:985-1000. [PubMed]
- McDonald DM, Choyke PL. Imaging of angiogenesis: from microscope to clinic. Nat Med 2003;9:713-25. [PubMed]
- Winkler F, Kozin SV, Tong RT, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell 2004;6:553-63. [PubMed]
- Heldin CH, Rubin K, Pietras K, et al. High interstitial fluid pressure - an obstacle in cancer therapy. Nat Rev Cancer 2004;4:806-13. [PubMed]
- Tacker JR, Anderson RU. Delivery of antitumor drug to bladder cancer by use of phase transition liposomes and hyperthermia. J Urol 1982;127:1211-4. [PubMed]
- Lorenzato C, Cernicanu A, Meyre ME, et al. MRI contrast variation of thermosensitive magnetoliposomes triggered by focused ultrasound: a tool for image-guided local drug delivery. Contrast Media Mol Imaging 2013;8:185-92. [PubMed]
- Escoffre JM, Mannaris C, Geers B, et al. Doxorubicin liposome-loaded microbubbles for contrast imaging and ultrasound-triggered drug delivery. IEEE Trans Ultrason Ferroelectr Freq Control 2013;60:78-87. [PubMed]
- Luan Y, Faez T, Gelderblom E, et al. Acoustical properties of individual liposome-loaded microbubbles. Ultrasound Med Biol 2012;38:2174-85. [PubMed]
- Mannaris C, Efthymiou E, Meyre ME, et al. In vitro localized release of thermosensitive liposomes with ultrasound-induced hyperthermia. Ultrasound Med Biol 2013;39:2011-20. [PubMed]
- Grüll H, Langereis S. Hyperthermia-triggered drug delivery from temperature-sensitive liposomes using MRI-guided high intensity focused ultrasound. J Control Release 2012;161:317-27. [PubMed]
- Geers B, Lentacker I, Sanders NN, et al. Self-assembled liposome-loaded microbubbles: The missing link for safe and efficient ultrasound triggered drug-delivery. J Control Release 2011;152:249-56. [PubMed]
- Böhmer MR, Chlon CH, Raju BI, et al. Focused ultrasound and microbubbles for enhanced extravasation. J Control Release 2010;148:18-24. [PubMed]
- Gramiak R, Shah PM. Echocardiography of the aortic root. Invest Radiol 1968;3:356-66. [PubMed]
- Weinstein JN, Magin RL, Yatvin MB, et al. Liposomes and local hyperthermia: selective delivery of methotrexate to heated tumors. Science 1979;204:188-91. [PubMed]
- Ta T, Porter TM. Thermosensitive liposomes for localized delivery and triggered release of chemotherapy. J Control Release 2013;169:112-25. [PubMed]
- Schroeder A, Kost J, Barenholz Y. Ultrasound, liposomes, and drug delivery: principles for using ultrasound to control the release of drugs from liposomes. Chem Phys Lipids 2009;162:1-16. [PubMed]
- Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev 2013;65:36-48. [PubMed]
- Weinstein JN, Magin RL, Yatvin MB, et al. Liposomes and local hyperthermia: selective delivery of methotrexate to heated tumors. Science 1979;204:188-91. [PubMed]
- Barenholz Y. Doxil®--the first FDA-approved nano-drug: lessons learned. J Control Release 2012;160:117-34. [PubMed]
- Al-Ahmady ZS, Al-Jamal WT, Bossche JV, et al. Lipid-peptide vesicle nanoscale hybrids for triggered drug release by mild hyperthermia in vitro and in vivo. ACS Nano 2012;6:9335-46. [PubMed]
- Faez T, Emmer M, Kooiman K, et al. 20 years of ultrasound contrast agent modeling. IEEE Trans Ultrason Ferroelectr Freq Control 2013;60:7-20. [PubMed]
- Hernot S, Unnikrishnan S, Du Z, et al. Nanobody-coupled microbubbles as novel molecular tracer. J Control Release 2012;158:346-53. [PubMed]
- Kooiman K, Vos HJ, Versluis M, et al. Acoustic behavior of microbubbles and implications for drug delivery. Adv Drug Deliv Rev 2014;72:28-48. [PubMed]
- Lentacker I, Lentacker I, Smedt S, et al. Drug loaded microbubble design for ultrasound triggered delivery. Soft Matter 2009;1-10.
- Fokong S, Theek B, Wu Z, et al. Image-guided, targeted and triggered drug delivery to tumors using polymer-based microbubbles. J Control Release 2012;163:75-81. [PubMed]
- Lentacker I, Geers B, Demeester J, et al. Design and evaluation of doxorubicin-containing microbubbles for ultrasound-triggered doxorubicin delivery: cytotoxicity and mechanisms involved. Mol Ther 2010;18:101-8. [PubMed]
- Geers B, Dewitte H, De Smedt SC, et al. Crucial factors and emerging concepts in ultrasound-triggered drug delivery. J Control Release 2012;164:248-55. [PubMed]
- Suzuki R, Takizawa T, Negishi Y, et al. Gene delivery by combination of novel liposomal bubbles with perfluoropropane and ultrasound. J Control Release 2007;117:130-6. [PubMed]
- Gao Z, Fain HD, Rapoport N. Ultrasound-enhanced tumor targeting of polymeric micellar drug carriers. Mol Pharm 2004;1:317-30. [PubMed]
- Somaglino L, Bouchoux G, Mestas JL, et al. Validation of an acoustic cavitation dose with hydroxyl radical production generated by inertial cavitation in pulsed mode: application to in vitro drug release from liposomes. Ultrason Sonochem 2011;18:577-88. [PubMed]
- Oerlemans C, Deckers R, Storm G, et al. Evidence for a new mechanism behind HIFU-triggered release from liposomes. J Control Release 2013;168:327-33. [PubMed]
- Sheeran PS, Wong VP, Luois S, et al. Decafluorobutane as a phase-change contrast agent for low-energy extravascular ultrasonic imaging. Ultrasound Med Biol 2011;37:1518-30. [PubMed]
- Fabiilli ML, Haworth KJ, Sebastian IE, et al. Delivery of chlorambucil using an acoustically-triggered perfluoropentane emulsion. Ultrasound Med Biol 2010;36:1364-75. [PubMed]
- Couture O, Faivre M, Pannacci N, et al. Ultrasound internal tattooing. Med Phys 2011;38:1116-23. [PubMed]
- Shpak O, Verweij M, Vos HJ, et al. Acoustic droplet vaporization is initiated by superharmonic focusing. Proc Natl Acad Sci U S A 2014;111:1697-702. [PubMed]
- McDannold N, Vykhodtseva N, Raymond S, et al. MRI-guided targeted blood-brain barrier disruption with focused ultrasound: histological findings in rabbits. Ultrasound Med Biol 2005;31:1527-37. [PubMed]
- Couture O, Urban A, Bretagne A, et al. In vivo targeted delivery of large payloads with an ultrasound clinical scanner. Med Phys 2012;39:5229-37. [PubMed]
- Kawabata K, Sugita N, Yoshikawa H, et al. Nanoparticles with Multiple Perfluorocarbons for Controllable Ultrasonically Induced Phase Shifting. Jpn J Appl Phys 2005;44:4548-52.
- Javadi M, Pitt WG, Belnap DM, et al. Encapsulating nanoemulsions inside eLiposomes for ultrasonic drug delivery. Langmuir 2012;28:14720-9. [PubMed]
- Couture O, Pannacci N, Babataheri A, et al. Ultrasound-inducible fluorescent particles for internal tattooing. Ultrasonics Symposium (IUS), IEEE International 2009;85-8.
- Fabiilli ML, Lee JA, Kripfgans OD, et al. Delivery of water-soluble drugs using acoustically triggered perfluorocarbon double emulsions. Pharm Res 2010;27:2753-65. [PubMed]
- Hernot S, Klibanov AL. Microbubbles in ultrasound-triggered drug and gene delivery. Adv Drug Deliv Rev 2008;60:1153-66. [PubMed]
- Owen J, Pankhurst Q, Stride E. Magnetic targeting and ultrasound mediated drug delivery: benefits, limitations and combination. Int J Hyperthermia 2012;28:362-73. [PubMed]
- Zhao S, Borden M, Bloch SH, et al. Radiation-force assisted targeting facilitates ultrasonic molecular imaging. Mol Imaging 2004;3:135-48. [PubMed]
- Fabiilli ML, Piert MR, Koeppe RA, et al. Assessment of the biodistribution of an [(18) F]FDG-loaded perfluorocarbon double emulsion using dynamic micro-PET in rats. Contrast Media Mol Imaging 2013;8:366-74. [PubMed]
- Maeda H. Macromolecular therapeutics in cancer treatment: the EPR effect and beyond. J Control Release 2012;164:138-44. [PubMed]
- Rapoport N. Ultrasound-mediated micellar drug delivery. Int J Hyperthermia 2012;28:374-85. [PubMed]
- Bassett JB, Anderson RU, Tacker JR. Use of temperature-sensitive liposomes in the selective delivery of methotrexate and cis-platinum analogues to murine bladder tumor. J Urol 1986;135:612-5. [PubMed]
- Koning GA, Eggermont AM, Lindner LH, et al. Hyperthermia and thermosensitive liposomes for improved delivery of chemotherapeutic drugs to solid tumors. Pharm Res 2010;27:1750-4. [PubMed]
- Rapoport NY, Kennedy AM, Shea JE, et al. Controlled and targeted tumor chemotherapy by ultrasound-activated nanoemulsions/microbubbles. J Control Release 2009;138:268-76. [PubMed]
- Mo S, Coussios CC, Seymour L, et al. Ultrasound-enhanced drug delivery for cancer. Expert Opin Drug Deliv 2012;9:1525-38. [PubMed]
- Dromi S, Frenkel V, Luk A, et al. Pulsed-high intensity focused ultrasound and low temperature-sensitive liposomes for enhanced targeted drug delivery and antitumor effect. Clin Cancer Res 2007;13:2722-7. [PubMed]
- Frenkel V, Etherington A, Greene M, et al. Delivery of liposomal doxorubicin (Doxil) in a breast cancer tumor model: investigation of potential enhancement by pulsed-high intensity focused ultrasound exposure. Acad Radiol 2006;13:469-79. [PubMed]
- Ning S, Macleod K, Abra RM, et al. Hyperthermia induces doxorubicin release from long-circulating liposomes and enhances their anti-tumor efficacy. Int J Radiat Oncol Biol Phys 1994;29:827-34. [PubMed]
- Ranjan A, Jacobs GC, Woods DL, et al. Image-guided drug delivery with magnetic resonance guided high intensity focused ultrasound and temperature sensitive liposomes in a rabbit Vx2 tumor model. J Control Release 2012;158:487-94. [PubMed]
- Kheirolomoom A, Lai CY, Tam SM, et al. Complete regression of local cancer using temperature-sensitive liposomes combined with ultrasound-mediated hyperthermia. J Control Release 2013;172:266-73. [PubMed]
- Lentacker I, Geers B, Demeester J, et al. Design and evaluation of doxorubicin-containing microbubbles for ultrasound-triggered doxorubicin delivery: cytotoxicity and mechanisms involved. Mol Ther 2010;18:101-8. [PubMed]
- Tinkov S, Coester C, Serba S, et al. New doxorubicin-loaded phospholipid microbubbles for targeted tumor therapy: in-vivo characterization. J Control Release 2010;148:368-72. [PubMed]
- Staples BJ, Pitt WG, Roeder BL, et al. Distribution of doxorubicin in rats undergoing ultrasonic drug delivery. J Pharm Sci 2010;99:3122-31. [PubMed]
- Cochran MC, Eisenbrey J, Ouma RO, et al. Doxorubicin and paclitaxel loaded microbubbles for ultrasound triggered drug delivery. Int J Pharm 2011;414:161-70. [PubMed]
Targeted Chemotherapy Using Focused Ultrasound for Liver Metastases (TARDOX) - Rychak JJ, Klibanov AL. Nucleic acid delivery with microbubbles and ultrasound Adv Drug Deliv Rev 2014;72:82-93. [J]. [PubMed]
- Unger E, Porter T, Lindner J, et al. Cardiovascular drug delivery with ultrasound and microbubbles. Adv Drug Deliv Rev 2014;72:110-26. [PubMed]
- Greenleaf WJ, Bolander ME, Sarkar G, et al. Artificial cavitation nuclei significantly enhance acoustically induced cell transfection. Ultrasound Med Biol 1998;24:587-95. [PubMed]
- Unger EC, McCreery TP, Sweitzer RH. Ultrasound enhances gene expression of liposomal transfection. Invest Radiol 1997;32:723-7. [PubMed]
- Anwer K, Kao G, Proctor B, et al. Ultrasound enhancement of cationic lipid-mediated gene transfer to primary tumors following systemic administration. Gene Ther 2000;7:1833-9. [PubMed]
- Negishi Y, Endo Y, Fukuyama T, et al. Delivery of siRNA into the cytoplasm by liposomal bubbles and ultrasound. J Control Release 2008;132:124-30. [PubMed]
- Carson AR, McTiernan CF, Lavery L, et al. Ultrasound-targeted microbubble destruction to deliver siRNA cancer therapy. Cancer Res 2012;72:6191-9. [PubMed]
- Carson AR, McTiernan CF, Lavery L, et al. Gene therapy of carcinoma using ultrasound-targeted microbubble destruction. Ultrasound Med Biol 2011;37:393-402. [PubMed]
- Wang DS, Panje C, Pysz MA, et al. Cationic versus neutral microbubbles for ultrasound-mediated gene delivery in cancer. Radiology 2012;264:721-32. [PubMed]
- Bazan-Peregrino M, Rifai B, Carlisle RC, et al. Cavitation-enhanced delivery of a replicating oncolytic adenovirus to tumors using focused ultrasound. J Control Release 2013;169:40-7. [PubMed]
- Unga J, Hashida M. Ultrasound induced cancer immunotherapy. Adv Drug Deliv Rev 2014;72:144-53. [PubMed]
- Xing Y, Lu X, Pua EC, et al. The effect of high intensity focused ultrasound treatment on metastases in a murine melanoma model. Biochem Biophys Res Commun 2008;375:645-50. [PubMed]
- Zhang Y, Deng J, Feng J, et al. Enhancement of antitumor vaccine in ablated hepatocellular carcinoma by high-intensity focused ultrasound. World J Gastroenterol 2010;16:3584-91. [PubMed]
- De Temmerman ML, Dewitte H, Vandenbroucke RE, et al. mRNA-Lipoplex loaded microbubble contrast agents for ultrasound-assisted transfection of dendritic cells. Biomaterials 2011;32:9128-35. [PubMed]
- Un K, Kawakami S, Suzuki R, et al. Development of an ultrasound-responsive and mannose-modified gene carrier for DNA vaccine therapy. Biomaterials 2010;31:7813-26. [PubMed]
- Bezagu M, Errico C, Chaulot-Talmon V, et al. High spatiotemporal control of spontaneous reactions using ultrasound-triggered composite droplets. J Am Chem Soc 2014;136:7205-8. [PubMed]
- Pardridge WM. Drug transport across the blood-brain barrier. Journal of Cerebral Blood Flow & Metabolism 2012;32:1959-72. [PubMed]
- Gaillard PJ, Visser CC, de Boer M, et al. Blood-to-brain drug delivery using nanocarriers. In: Hammarlund-Udenaes M, de Lange E, Thorne RG, et al. eds. Drug Delivery to the Brain. Berlin: Springer, 2014;433-54.
- Hammarlund-Udenaes M, de Lange E, Thorne RG, et al. eds. Drug Delivery to the Brain. Berlin: Springer, 2014.
- Hynynen K, McDannold N, Vykhodtseva N, et al. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology 2001;220:640-6. [PubMed]
- Aryal M, Arvanitis CD, Alexander PM, et al. Ultrasound-mediated blood-brain barrier disruption for targeted drug delivery in the central nervous system. Adv Drug Deliv Rev 2014;72:94-109. [PubMed]
- Tachibana K, Feril LB Jr, Ikeda-Dantsuji Y. Sonodynamic therapy. Ultrasonics 2008;48:253-9. [PubMed]
- Marty B, Larrat B, Van Landeghem M, et al. Dynamic study of blood-brain barrier closure after its disruption using ultrasound: a quantitative analysis. J Cereb Blood Flow Metab 2012;32:1948-58. [PubMed]
- Kinoshita M, McDannold N, Jolesz FA, et al. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proc Natl Acad Sci U S A 2006;103:11719-23. [PubMed]
- Treat LH, McDannold N, Vykhodtseva N, et al. Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound. Int J Cancer 2007;121:901-7. [PubMed]
- Mei J, Cheng Y, Song Y, et al. Experimental study on targeted methotrexate delivery to the rabbit brain via magnetic resonance imaging-guided focused ultrasound. J Ultrasound Med 2009;28:871-80. [PubMed]
- Zeng HQ, Lü L, Wang F, et al. Focused ultrasound-induced blood-brain barrier disruption enhances the delivery of cytarabine to the rat brain. J Chemother 2012;24:358-63. [PubMed]
- Park J, Zhang Y, Vykhodtseva N, et al. The kinetics of blood brain barrier permeability and targeted doxorubicin delivery into brain induced by focused ultrasound. J Control Release 2012;162:134-42. [PubMed]
- Huang Q, Deng J, Wang F, et al. Targeted gene delivery to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Exp Neurol 2012;233:350-6. [PubMed]
- Thévenot E, Jordão JF, O’Reilly MA, et al. Targeted delivery of self-complementary adeno-associated virus serotype 9 to the brain, using magnetic resonance imaging-guided focused ultrasound. Hum Gene Ther 2012;23:1144-55. [PubMed]
- Alonso A, Reinz E, Leuchs B, et al. Focal Delivery of AAV2/1-transgenes Into the Rat Brain by Localized Ultrasound-induced BBB Opening. Mol Ther Nucleic Acids 2013;2:e73 [PubMed]
- Hsu PH, Wei KC, Huang CY, et al. Noninvasive and targeted gene delivery into the brain using microbubble-facilitated focused ultrasound. PLoS One 2013;8:e57682 [PubMed]
- Liu HL, Fan CH, Ting CY, et al. Combining microbubbles and ultrasound for drug delivery to brain tumors: current progress and overview. Theranostics 2014;4:432-44. [PubMed]
- Kovacs Z, Werner B, Rassi A, et al. Prolonged survival upon ultrasound-enhanced doxorubicin delivery in two syngenic glioblastoma mouse models. J Control Release 2014;187:74-82. [PubMed]
- Burke CW, Alexander E 4th, Timbie K, et al. Ultrasound-activated agents comprised of 5FU-bearing nanoparticles bonded to microbubbles inhibit solid tumor growth and improve survival. Mol Ther 2014;22:321-8. [PubMed]
- Aryal M, Vykhodtseva N, Zhang YZ, et al. Multiple treatments with liposomal doxorubicin and ultrasound-induced disruption of blood-tumor and blood-brain barriers improve outcomes in a rat glioma model. J Control Release 2013;169:103-11. [PubMed]
- Wei KC, Chu PC, Wang HY, et al. Focused ultrasound-induced blood-brain barrier opening to enhance temozolomide delivery for glioblastoma treatment: a preclinical study. PloS one 2013;8:e58995 [PubMed]
- Fan CH, Ting CY, Liu HL, et al. Antiangiogenic-targeting drug-loaded microbubbles combined with focused ultrasound for glioma treatment. Biomaterials 2013;34:2142-55. [PubMed]
- Fan CH, Ting CY, Lin HJ, et al. SPIO-conjugated, doxorubicin-loaded microbubbles for concurrent MRI and focused-ultrasound enhanced brain-tumor drug delivery. Biomaterials 2013;34:3706-15. [PubMed]
- Alkins R, Burgess A, Ganguly M, et al. Focused ultrasound delivers targeted immune cells to metastatic brain tumors. Cancer Res 2013;73:1892-9. [PubMed]
- Alkins RD, Brodersen PM, Sodhi RN, et al. Enhancing drug delivery for boron neutron capture therapy of brain tumors with focused ultrasound. Neuro Oncol 2013;15:1225-35. [PubMed]
- Park EJ, Zhang YZ, Vykhodtseva N, et al. Ultrasound-mediated blood-brain/blood-tumor barrier disruption improves outcomes with trastuzumab in a breast cancer brain metastasis model. J Control Release 2012;163:277-84. [PubMed]
- Treat LH, McDannold N, Zhang Y, et al. Improved anti-tumor effect of liposomal doxorubicin after targeted blood-brain barrier disruption by MRI-guided focused ultrasound in rat glioma. Ultrasound Med Biol 2012;38:1716-25. [PubMed]
- Yang FY, Wong TT, Teng MC, et al. Focused ultrasound and interleukin-4 receptor-targeted liposomal doxorubicin for enhanced targeted drug delivery and antitumor effect in glioblastoma multiforme. J Control Release 2012;160:652-8. [PubMed]
- Ting CY, Fan CH, Liu HL, et al. Concurrent blood-brain barrier opening and local drug delivery using drug-carrying microbubbles and focused ultrasound for brain glioma treatment. Biomaterials 2012;33:704-12. [PubMed]
- Yang FY, Chen YW, Chou FI, et al. Boron neutron capture therapy for glioblastoma multiforme: enhanced drug delivery and antitumor effect following blood-brain barrier disruption induced by focused ultrasound. Future Oncol 2012;8:1361-9. [PubMed]
- Liu HL, Hua MY, Chen PY, et al. Blood-Brain Barrier Disruption with Focused Ultrasound Enhances Delivery of Chemotherapeutic Drugs for Glioblastoma Treatment 1. Radiology 2010;255:415-25. [PubMed]
- Liu HL, Hua MY, Yang HW, et al. Magnetic resonance monitoring of focused ultrasound/magnetic nanoparticle targeting delivery of therapeutic agents to the brain. Proc Natl Acad Sci U S A 2010;107:15205-10. [PubMed]
- Chen PY, Liu HL, Hua MY, et al. Novel magnetic/ultrasound focusing system enhances nanoparticle drug delivery for glioma treatment. Neuro Oncol 2010;12:1050-60. [PubMed]
- Yang FY, Teng MC, Lu M, et al. Treating glioblastoma multiforme with selective high-dose liposomal doxorubicin chemotherapy induced by repeated focused ultrasound. Int J Nanomedicine 2012;7:965-74. [PubMed]
- McDannold N, Zhang Y, Vykhodtseva N. Blood-brain barrier disruption and vascular damage induced by ultrasound bursts combined with microbubbles can be influenced by choice of anesthesia protocol. Ultrasound Med Biol 2011;37:1259-70. [PubMed]
- Delalande A, Postema M, Mignet N, et al. Ultrasound and microbubble-assisted gene delivery: recent advances and ongoing challenges. Ther Deliv 2012;3:1199-215. [PubMed]
- Mullin LB, Phillips LC, Dayton PA. Nanoparticle delivery enhancement with acoustically activated microbubbles. IEEE Trans Ultrason Ferroelectr Freq Control 2013;60:65-77. [PubMed]
- Lentacker I, De Cock I, Deckers R, et al. Understanding ultrasound induced sonoporation: definitions and underlying mechanisms. Adv Drug Deliv Rev 2014;72:49-64. [PubMed]
- Bao S, Thrall BD, Gies RA, et al. In vivo transfection of melanoma cells by lithotripter shock waves. Cancer Res 1998;58:219-21. [PubMed]
- Miller DL, Bao S, Gies RA, et al. Ultrasonic enhancement of gene transfection in murine melanoma tumors. Ultrasound Med Biol 1999;25:1425-30. [PubMed]
- Manome Y, Nakamura M, Ohno T, et al. Ultrasound facilitates transduction of naked plasmid DNA into colon carcinoma cells in vitro and in vivo. Hum Gene Ther 2000;11:1521-8. [PubMed]
- Anwer K, Kao G, Proctor B, et al. Ultrasound enhancement of cationic lipid-mediated gene transfer to primary tumors following systemic administration. Gene Ther 2000;7:1833-9. [PubMed]
- Song J, Tata D, Li L, et al. Combined shock-wave and immunogene therapy of mouse melanoma and renal carcinoma tumors. Ultrasound Med Biol 2002;28:957-64. [PubMed]
- Hayashi S, Mizuno M, Yoshida J, et al. Effect of sonoporation on cationic liposome-mediated IFNbeta gene therapy for metastatic hepatic tumors of murine colon cancer. Cancer Gene Ther 2009;16:638-43. [PubMed]
- Casey G, Cashman JP, Morrissey D, et al. Sonoporation mediated immunogene therapy of solid tumors. Ultrasound Med Biol 2010;36:430-40. [PubMed]
- Miller DL, Song J. Tumor growth reduction and DNA transfer by cavitation-enhanced high-intensity focused ultrasound in vivo. Ultrasound Med Biol 2003;29:887-93. [PubMed]
- Sakakima Y, Hayashi S, Yagi Y, et al. Gene therapy for hepatocellular carcinoma using sonoporation enhanced by contrast agents. Cancer Gene Ther 2005;12:884-9. [PubMed]
- Hauff P, Seemann S, Reszka R, et al. Evaluation of gas-filled microparticles and sonoporation as gene delivery system: feasibility study in rodent tumor models. Radiology 2005;236:572-8. [PubMed]
- Li YS, Davidson E, Reid CN, et al. Optimising ultrasound-mediated gene transfer (sonoporation) in vitro and prolonged expression of a transgene in vivo: potential applications for gene therapy of cancer. Cancer Lett 2009;273:62-9. [PubMed]
- Tsai KC, Liao ZK, Yang SJ, et al. Differences in gene expression between sonoporation in tumor and in muscle. J Gene Med 2009;11:933-40. [PubMed]
- Suzuki R, Namai E, Oda Y, et al. Cancer gene therapy by IL-12 gene delivery using liposomal bubbles and tumoral ultrasound exposure. J Control Release 2010;142:245-50. [PubMed]
- Zolochevska O, Xia X, Williams BJ, et al. Sonoporation delivery of interleukin-27 gene therapy efficiently reduces prostate tumor cell growth in vivo. Hum Gene Ther 2011;22:1537-50. [PubMed]
- Sirsi SR, Hernandez SL, Zielinski L, et al. Polyplex-microbubble hybrids for ultrasound-guided plasmid DNA delivery to solid tumors. J Control Release 2012;157:224-34. [PubMed]
- Liao ZK, Tsai KC, Wang HT, et al. Sonoporation-mediated anti-angiogenic gene transfer into muscle effectively regresses distant orthotopic tumors. Cancer Gene Ther 2012;19:171-80. [PubMed]
- Kono Y, Kawakami S, Higuchi Y, et al. Antitumor effect of nuclear factor-κB decoy transfer by mannose-modified bubble lipoplex into macrophages in mouse malignant ascites. Cancer Sci 2014;105:1049-55. [PubMed]
- Kono Y, Kawakami S, Higuchi Y, et al. Tumour-associated macrophages targeted transfection with NF-κB decoy/mannose-modified bubble lipoplexes inhibits tumour growth in tumour-bearing mice. J Drug Target 2014;22:439-49. [PubMed]
- Rychak JJ, Klibanov AL. Nucleic acid delivery with microbubbles and ultrasound. Adv Drug Deliv Rev 2014;72:82-93. [PubMed]
- Weiss N, Delius M, Gambihler S, et al. Effect of shock waves and cisplatin on cisplatin-sensitive and -resistant rodent tumors in vivo. Int J Cancer 1994;58:693-9. [PubMed]
- Hoshi S, Orikasa S, Suzuki K, et al. High-energy underwater shock wave treatment for internal iliac muscle metastasis of prostatic cancer: a first clinical trial. Jpn J Cancer Res 1995;86:424-8. [PubMed]
- Iwanaga K, Tominaga K, Yamamoto K, et al. Local delivery system of cytotoxic agents to tumors by focused sonoporation. Cancer Gene Ther 2007;14:354-63. [PubMed]
- Matsuo M, Yamaguchi K, Feril LB Jr, et al. Synergistic inhibition of malignant melanoma proliferation by melphalan combined with ultrasound and microbubbles. Ultrason Sonochem 2011;18:1218-24. [PubMed]
- Yamatomo N, Iwagami T, Kato I, et al. Sonoporation as an enhancing method for boron neutron capture therapy for squamous cell carcinomas. Radiat Oncol 2013;8:280. [PubMed]
- Sato T, Mori S, Arai Y, et al. The combination of intralymphatic chemotherapy with ultrasound and nano-/microbubbles is efficient in the treatment of experimental tumors in mouse lymph nodes. Ultrasound Med Biol 2014;40:1237-49. [PubMed]
- Kotopoulis S, Delalande A, Popa M, et al. Sonoporation-enhanced chemotherapy significantly reduces primary tumour burden in an orthotopic pancreatic cancer xenograft. Mol Imaging Biol 2014;16:53-62. [PubMed]
- Kotopoulis S, Dimcevski G, Gilja OH, et al. Treatment of human pancreatic cancer using combined ultrasound, microbubbles, and gemcitabine: a clinical case study. Med Phys 2013;40:072902 [PubMed]
- Yudina A, Lepetit-Coiffé M, De Smet M, et al. In vivo temperature controlled ultrasound-mediated intracellular delivery of cell-impermeable compounds. J Control Release 2012;161:90-7. [PubMed]
- Robertson CA, Evans DH, Abrahamse H. Photodynamic therapy (PDT): a short review on cellular mechanisms and cancer research applications for PDT. J Photochem Photobiol B 2009;96:1-8. [PubMed]
- O’Connor AE, Gallagher WM, Byrne AT. Porphyrin and nonporphyrin photosensitizers in oncology: preclinical and clinical advances in photodynamic therapy. Photochem Photobiol 2009;85:1053-74. [PubMed]
- Triesscheijn M, Baas P, Schellens JH, et al. Photodynamic therapy in oncology. Oncologist 2006;11:1034-44. [PubMed]
- Nielsen KP, Juzeniene A, Juzenas P, et al. Choice of optimal wavelength for PDT: the significance of oxygen depletion. Photochem Photobiol 2005;81:1190-4. [PubMed]
- Moriwaki SI, Misawa J, Yoshinari Y, et al. Analysis of photosensitivity in Japanese cancer-bearing patients receiving photodynamic therapy with porfimer sodium (Photofrin). Photodermatol Photoimmunol Photomed 2001;17:241-3. [PubMed]
- Ohmura T, Fukushima T, Shibaguchi H, et al. Sonodynamic therapy with 5-aminolevulinic acid and focused ultrasound for deep-seated intracranial glioma in rat. Anticancer Res 2011;31:2527-33. [PubMed]
- Nonaka M, Yamamoto M, Yoshino S, et al. Sonodynamic therapy consisting of focused ultrasound and a photosensitizer causes a selective antitumor effect in a rat intracranial glioma model. Anticancer Res 2009;29:943-50. [PubMed]
- Tang W, Liu Q, Wang X, et al. Potential mechanism in sonodynamic therapy and focused ultrasound induced apoptosis in sarcoma 180 cells in vitro. Ultrasonics 2009;49:786-93. [PubMed]
- Wang X, Wang P, Zhang K, et al. Initiation of autophagy and apoptosis by sonodynamic therapy in murine leukemia L1210 cells. Toxicol In Vitro 2013;27:1247-59. [PubMed]
- Su X, Wang P, Wang X, et al. Apoptosis of U937 cells induced by hematoporphyrin monomethyl ether-mediated sonodynamic action. Cancer Biother Radiopharm 2013;28:207-17. [PubMed]
- Umemura S, Yumita N, Nishigaki R, et al. Mechanism of cell damage by ultrasound in combination with hematoporphyrin. Jpn J Cancer Res 1990;81:962-6. [PubMed]
- Shibaguchi H, Tsuru H, Kuroki M, et al. Sonodynamic cancer therapy: a non-invasive and repeatable approach using low-intensity ultrasound with a sonosensitizer. Anticancer Res 2011;31:2425-9. [PubMed]
- Trendowski M. The promise of sonodynamic therapy. Cancer Metastasis Rev 2014;33:143-60. [PubMed]
- Chen H, Zhou X, Gao Y, et al. Recent progress in development of new sonosensitizers for sonodynamic cancer therapy. Drug Discov Today 2014;19:502-9. [PubMed]
- Kim J, Chung DJ, Jung SE, et al. Therapeutic effect of high-intensity focused ultrasound combined with transarterial chemoembolisation for hepatocellular carcinoma <5 cm: comparison with transarterial chemoembolisation monotherapy--preliminary observations. Br J Radiol 2012;85:e940-6. [PubMed]
- Li C, Zhang W, Zhang R, et al. Therapeutic effects and prognostic factors in high-intensity focused ultrasound combined with chemoembolisation for larger hepatocellular carcinoma. Eur J Cancer 2010;46:2513-21. [PubMed]
- Finley DS, Pouliot F, Shuch B, et al. Ultrasound-based combination therapy: potential in urologic cancer. Expert Rev Anticancer Ther 2011;11:107-13. [PubMed]
- Wang S, Zderic V, Frenkel V. Extracorporeal, low-energy focused ultrasound for noninvasive and nondestructive targeted hyperthermia. Future Oncol 2010;6:1497-511. [PubMed]
- Kampinga HH. Cell biological effects of hyperthermia alone or combined with radiation or drugs: a short introduction to newcomers in the field. Int J Hyperthermia 2006;22:191-6. [PubMed]
- Diaz RJ, McVeigh PZ, O’Reilly MA, et al. Focused ultrasound delivery of Raman nanoparticles across the blood-brain barrier: potential for targeting experimental brain tumors. Nanomedicine 2014;10:1075-87. [PubMed]


