
Neo-adjuvant chemotherapy followed by radical resection for primary borderline resectable unicentric Castleman disease: a case report
Introduction
Castleman disease, firstly described in 1954 by Benjamin Castleman, is a group of infrequent benign disorders with the histological trait of lymphatic tissue hyperplasia (1). There are two anatomical subtypes for CD: unicentric Castleman disease (UCD), which confines to one lymphatic region and multicentric Castleman disease (MCD), which presents disease within multiple lymphatic areas (2). Two histopathological subtypes were classified: hyaline vascular type of CD (HVCD) and plasma cell type of CD (PCCD) (3). As a heterogeneous disorder, CD can show completely different symptoms. Most of UCD patients have symptoms associated with surrounding tissue compressed by the tumor. Besides, PCCD is often accompanied by systemic symptoms, such as pyrexia, fatigue, anemia and weight loss due to the persistent over-releasing cytokines like interleukin (IL)-6 (2). Surgery is the curative treatment for UCD when possible. Systemic treatment is generally the first selection of MCD. However, for the unresectable or borderline resectable UCD, no standard treatment was established (4). In the present study, we introduce a successful case showing neo-adjuvant chemotherapy followed by radical resection for a borderline resectable UCD. We present the following case in accordance with the CARE Guideline (5).
Case presentation
A 28-year-old male with a 2-month history of fatigue, dizziness and weight loss was referred to surgery department due to the retroperitoneal mass revealed by physical examination. The patient had a history of chronic hepatitis B for 20 years and took Entecavir for 4 months (Figure 1). Computed tomography (CT) imaging of the abdomen exhibited a retroperitoneal tumor with the size of 77 mm ×46 mm ×85 mm abutting inferior vena cava and hepatic portal (Figure 1A). Ultrasound guided fine needle biopsy suggested the diagnosis of Castleman disease, plasma cell subtype, which expressed cluster of differentiation 20 (CD20) (Figure 1B). Subsequent positron emission tomography/computed tomography (PET/CT) confirmed it was a unicentric disease, with no other lymph nodes affected. Since the location of this large and hypervascular lesion was close to hepatic portal and compressed or adhered to the major vessels, primary operation was considered high risk and borderline resectable. Thus, we held a multidisciplinary team (MDT) meeting and decided to start neo-adjuvant chemotherapy to try to downsize the tumor before the surgery. No contraindications of chemotherapy were revealed and the 6-cycle R-CHOP (intravenous rituximab 375 mg/m2 on day 1, intravenous cyclophosphamide 720 mg/m2, intravenous epirubicin 70 mg/m2, intravenous vincristine 2 mg (maximum total of 2 mg) on day 2, and oral prednisone 30 mg on day 2–6) chemotherapy regimens would be executed every three weeks (Table 1).
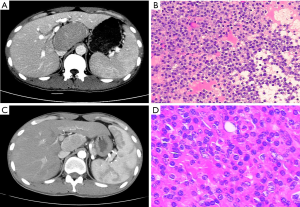
Table 1
| Time | Therapy | Status |
|---|---|---|
| Week 1–3 | First R-CHOP chemotherapy | – |
| Day 1 | intravenous rituximab 375 mg/m2 | – |
| Day 2 | intravenous cyclophosphamide 720 mg/m2, intravenous epirubicin 70 mg/m2, intravenous vincristine 2 mg, oral prednisone 30 mg | – |
| Day 3–6 | oral prednisone 30 mg | – |
| Week 4–18 | Other 5 cycle R-CHOP chemotherapy every 3 weeks | Tumor shrank and vascular compression relieved |
| Week 19 | laparoscopic tumor resection | – |
| 1-year follow-up | – | No recurrence |
The patient decided to receive the neo-adjuvant chemotherapy and volunteered for the risks and side effects of chemotherapy. After finishing 18 weeks of the chemotherapy, the tumor was shrinked, with the size of 32 mm ×50 mm ×55 mm (Figure 1C). The compressive effect for the major vessels was significantly relieved as shown by CT scan and patient’s condition of anemia and hypoalbuminemia was improved. Then a laparoscopic retroperitoneal tumor resection was performed successfully and the postoperative pathological examination gave a same diagnosis of Castleman disease, plasma cell subtype (Figure 1D). During 1-year follow-up, no recurrence signs have been detected by image examination. No symptoms of fatigue or dizziness exist.
Discussion
Radical surgical resection is the preferred option for UCD, with 10-year overall survival rates exceeding 95% (6). However, there is no standard therapy for unresectable or borderline resectable UCD. Recently, a variety of systemic strategies were reported for treating unresectable UCD, including anti-inflammatory and immunosuppressive therapy, cytotoxic chemotherapy, radiotherapy and biological targeted therapy (2). However, all of these single treatments showed some efficacy but could not achieve curative outcome. To overcome this problem, de Vries IA and colleges conducted neo-adjuvant radiotherapy in an unresectable UCD, HV subtype, and finally accomplished radical surgery resection (7). Similarly, a primary unresectable thoracic UCD, HV subtype, was successfully downsized and surgically removed after neo-adjuvant rituximab therapy, which is a monoclonal antibody against CD20 (8). In the present case, since the tumor was huge, radiotherapy was rejected with the concern of surrounding organ damage when the target volume was too large. Instead, rituximab in combination with CHOP regimen was chosen for neo-adjuvant therapy for this patient. Rituximab was used to destroy CD20 producing tumor cells. However, rituximab single treatment might not be sufficient for this case considering it was a PCCD, which is a more aggressive disease compared to HVCD, as shown in previous study (9). It should be pointed out that a combined chemotherapy regimen, known as CHOP (containing cyclophosphamide, epirubicin, vincristine and prednisone) was effective and was widely used in B-cell proliferative diseases including PCCD. Although there have been no randomized controlled studies, multiple clinical researches have evaluated the effect of CHOP with and without Rituximab for CD20 positive non-Hodgkin’s lymphoma and confirmed that the former one had observably better efficacy (10). In our case, which was a CD20 positive PCCD, rituximab in combination with CHOP chemotherapy regimen was also found to be effective for neo-adjuvant therapy. Although we observed good response in this patient and a radical, minimal invasive procedure was accomplished eventually, more clinical studies are needed to help better investigate the efficacy of adjuvant treatment as well as the individualized option of neo-adjuvant treatment for borderline resectable or unresectable UCD.
This study demonstrated that neo-adjuvant R-CHOP chemotherapy alleviated the symptom of PCCD as well as downsized the tumor to provide an opportunity for radical surgical resection. More studies will be needed in the future to clarify the role of neo-adjuvant treatment for borderline resectable or unresectable UCD.
Acknowledgments
Funding: The study was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.02.45). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this Case Report and any accompanying images. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Castleman B, Towne VW. Case records of the Massachusetts General Hospital; weekly clinicopathological exercises; founded by Richard C. Cabot. N Engl J Med 1954;251:396-400. [PubMed]
- Chan KL, Lade S, Prince HM, et al. Update and new approaches in the treatment of Castleman disease. J Blood Med 2016;7:145-58. [Crossref] [PubMed]
- Keller AR, Hochholzer L, Castleman B. Hyaline-vascular and plasma-cell types of giant lymph node hyperplasia of the mediastinum and other locations. Cancer. 1972;29:670-83. [Crossref] [PubMed]
- Soumerai JD, Sohani AR, Abramson JS. Diagnosis and management of Castleman disease. Cancer Control 2014;21:266-78. [Crossref] [PubMed]
- Riley DS, Barber MS, Kienle GS, et al. CARE 2013 Explanations and Elaborations: Reporting Guidelines for Case Reports. J Clin Epidemiol 2017;89:218-35. [Crossref] [PubMed]
- Talat N, Belgaumkar AP, Schulte KM. Surgery in Castleman's disease: a systematic review of 404 published cases. Ann Surg 2012;255:677-84. [Crossref] [PubMed]
- de Vries IA, van Acht MM, Demeyere T, et al. Neoadjuvant radiotherapy of primary irresectable unicentric Castleman’s disease: a case report and review of the literature. Radiat Oncol 2010;5:7. [Crossref] [PubMed]
- Bandera B, Ainsworth C, Shikle J, et al. Treatment of unicentric Castleman disease with neoadjuvant rituximab. Chest 2010;138:1239-41. [Crossref] [PubMed]
- Talat N, Schulte KM. Castleman's disease: systematic analysis of 416 patients from the literature. Oncologist 2011;16:1316-24. [Crossref] [PubMed]
- Sohn BS, Kim SM, Yoon DH, et al. The comparison between CHOP and R-CHOP in primary gastric diffuse large B cell lymphoma. Ann Hematol 2012;91:1731-9. [Crossref] [PubMed]


