An operated case of locally advanced thymic atypical carcinoid in anterior mediastinum: a case report
Introduction
Primary neuroendocrine tumors (NETs) only account for 2–4% of all mediastinal tumors but lead to poor prognosis (1). Thymic NETs are classified into thymic typical carcinoid (TTC), thymic atypical carcinoid (TAC), large cell neuroendocrine carcinoma (LCNEC) and small cell carcinoma (SCC) according to the 2015 WHO Classification of Tumors of the Thymus (1). Primary NETs of the mediastinum are rare (2). Thymic NETs are usually associated with endocrine disorders such as Cushing’s syndrome or the multiple endocrine neoplasms (MEN) type I syndrome (3,4). Thymic carcinoids have a poor prognosis owing to invasive behavior, distant metastasis and high rate of postoperative recurrence. More than 70% of these patients will develop local or distant metastasis within 5 years after diagnosis (5). Here we report a case of locally advanced TAC detected in the left-anterior mediastinum in an adult. After timely radical surgery and postoperative adjuvant chemotherapy, the patient is still alive without recurrence or metastasis. Therefore, radical resection with reginal lymph nodes dissection should be the first choice for locally advanced TAC patients. If the mediastinal mass is large and squeezes peripheral major vessels or heart, open surgery should be taken into consideration. We present the following case in accordance with the CARE-Guideline (6).
Case presentation
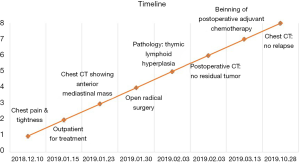
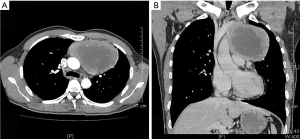
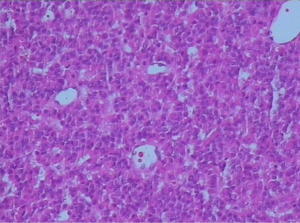
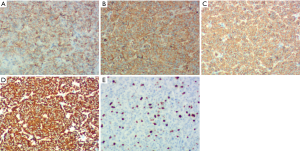
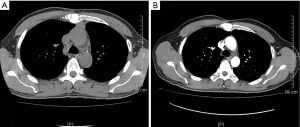
On January 15th in 2019, a 42-year-old male was referred to our hospital, complaining of chest pain and tightness for over 1 month, and the symptoms were getting worse gradually in the last week. He had no history of malignancy, and tumor family history, as well as genetic history, was not found. The patient reported no fever, cough, hemoptysis, difficulty swallowing, dyspnea, hoarseness and limb weakness. No positive signs were detected in routine physical examination and tumor markers were normal, such as carcino-embryonic antigen (CEA), ferritin (FER). Chest CT revealed a large left-anterior mediastinum mass. Further evaluation demonstrated a 115 mm × 95 mm mass with patchy necrosis, heterogeneous enhancement and small blood vessels. Moreover, the large mass compressed left brachiocephalic veins, pericardium and upper-lobe of left lung (Figure 1). Based on clinical and CT appearances, the patient was suspected of invasive thymoma or thymic carcinoma pre-operatively. Due to the estimated great-difficulty by video-assisted thoracoscopic surgery (VATS), median sternotomy was performed to remove the tumor in January 30th, 2019. During the surgery, we found that the tumor was located in the left-anterior mediastinum with incomplete capsule, and that part of upper-lobe of left lung was compressed by the large mass. Complete tumor resection with combined reginal mediastinal lymph node dissection were performed. Hematoxylin-eosin (HE) staining showed that the tumor cells were arranged in a nested, or ribbon pattern, with mild atypia, local hemorrhage, foci of necrosis and abundant interstitial blood vessels (Figure 2). Immunohistochemistry demonstrated positivity for CD56, Syn, CgA, CK (Figure 3A,B,C,D), and staining indices for Ki-67 was 15% (Figure 3E). No metastasis was found in all the 4 resected mediastinal lymph nodes. Postoperative pathology turned out to be primary TAC, Masaoka stage II (7). Postoperative chest CT (Figure 4A) showed that the tumor was resected completely and no recurrence occurred in postoperative follow-up (Figure 4B). From March 3rd on, a total of 6 cycles of postoperative adjuvant chemotherapy was given per 3 weeks successfully, and the chemotherapy regimen was gemcitabine (1,000 mg/m2 on 1st day) plus cisplatin (80 mg/m2 on 1st day). No 3/4 grade adverse effects (AEs) occurred during the perioperative period. There was no tumor recurrence or metastasis during the postoperative follow-up. A time lime showed the whole medical procedure of the special case (Figure 5).
Discussion
NETs have been reported in pancreas (8), gastrointestinal tract (9), lung (10), thymus, pituitary (11), thyroid (12), parathyroid, breast, skin, adrenal gland, paraganglia and genito-urinary system (13). In 1972, thymic carcinoid was first described by Rosai and Higa (14). So far, almost 220 TAC cases have been reported since then. Among all the NETs, TAC is relatively rare and often found by accident. It mainly occurs in men (15,16) and nearly one-third of patients with this malignancy are asymptomatic (17). Szolkowska et al. summarized that the percentage of thymic carcinoid in primary mediastinal neoplasms was nearly 1% (18). Thymic NETs originate from neuroendocrine cells, which are diffusely distributed in different tissues and organs. Primary TAC only represents a minority of NETs. However, prognosis of locally advanced primary TAC is not optimistic due to the high risk of recurrence and metastasis. And there are no accepted guidelines for us to deal with the rare disease. In this report, we presented a rare case of TAC located in left-anterior mediastinum in a middle-aged male. To our delight, the patient is still alive without local recurrence or metastasis after timely radical operation and post-operative adjuvant chemotherapy. However, no population-based data are available to build up guidelines for diagnosis, treatment, and follow-up.
Thymic carcinoids are more likely to have a large mass, irregular contour, heterogeneous intensity, heterogeneous enhancement and local invasion on CT or MRI (19,20). A necrotic or cystic component is often seen in atypical carcinoid. Small biopsy often cannot distinguish AC from TC, so surgical specimens are required to figure out precise classifications. Atypical carcinoid tumor is featured by a high degree of malignancy and invasiveness, and patients often complain of respiratory or local chest symptoms, such as cough, dyspnea, and chest pain. Thymic NETs are frequently associated with autoimmune disease or endocrine disorders, and the most common is Cushing syndrome, characterized by ectopic production of adrenocorticotropic hormone (ACTH) (21,22). The case in our study presented to hospital due to local chest symptoms, which may attribute to compression by the large mediastinal mass. Because of asymptomatic status at the early stage, a majority of patients were initially diagnosed at an advanced stage. Walts et al. (21) found that thymic NETs were usually large at high Masaoka stage at initial diagnosis and were associated with poor prognosis in spite of aggressive treatment.
It is generally accepted that strong and diffuse expression of more than one of four neuroendocrine markers (chromogranin A, synaptophysin, CD56 and NSE) in >50% of tumor cells has been maintained to diagnosed with NETs. Histopathologic characteristics of TAC include carcinoid morphology, 2–10 mitosis/2 mm2, or foci of necrosis. In addition, TAC is well differentiated and it is suggested as intermediate grade neuroendocrine tumor (23). Moreover, thymic NETs genetics differ from each other (24), so precision genetics analysis may provide new avenues for diagnosis and treatment for TAC in future. Treatment strategies for TAC mainly include surgery, chemotherapy, and radiotherapy. Due to its aggressive behaviour and lack of effective chemotherapy or radiotherapy, surgical approach may be the best treatment to deal with TAC. Complete resection is deemed to be a favorable prognostic factor for patients with thymic NETs (15-17), but the rate of postoperative recurrence is high. A retrospective study in Japan (25) found that distant metastasis in bone and lung tissues was more common than local recurrence. Araki et al. (26) revealed that the rate of recurrence or metastasis in thymic NETs patients was 79%, including intra-thoracic metastasis (thoracic lymph nodes, pleura, lung, pericardium, postoperative mediastinum) and extra-thoracic metastasis (bone, abdominal lymph nodes, liver, pancreas, kidney, adrenal gland, spleen, brain). This article concluded that metastasis, as well as recurrence, was frequent, mainly involving thoracic lymph nodes, but extra-thoracic metastasis may also happen. Wu et al. (27) reported a case presented with neck pain and right upper limb numbness, and the case was diagnosed with cervical vertebra metastasis resulting from TAC. Studies have revealed that postoperative adjuvant chemotherapy or radiotherapy can prolong survival time. In our article, the case underwent complete resection and have survived to the present day without recurrence or metastasis. We chose open thymectomy rather than VATs because open surgery may remove the tumor to the maximum possible extent. Also, postoperative adjuvant chemotherapy was regularly performed to reduce the rate of recurrence or metastasis.
A population-based study by Jennifer suggested that the median overall survival time was 73 months and the 5-year survival rate was 56% in 254 thymic NETs cases, and patients who underwent surgery had a significantly longer survival time than those without surgery (28). Tumor size, proliferation index, histologic type, surgical resection, the Masaoka staging and postoperative radiotherapy have an impact on prognosis (16,17,29). Han et al. (30) found that the number of mitosis was an independent prognostic factor for recurrence and death in patients with atypical carcinoid tumor of the lung and thymus. In the above study, all seven TAC patients underwent total thymectomy with neck lymph node dissection, and six of seven received adjuvant chemotherapy, radiotherapy or chemoradiotherapy, but recurrence/metastasis appeared only in one patient, whose recurrence sites appeared on the mediastinum, pleura, brain, and bone. To the best of our knowledge, radical surgery is very necessary to perform for those locally advanced TAC. However, the management of TAC is full of challenge and requires a multi-discipline team for the final diagnosis and treatment.
We suggest that all NETs need to have a comprehensive assessment after clear diagnosis. In the case of our report, combination of radical surgery and adjuvant chemotherapy provide an excellent alternative for locally advanced TAC patients. Due to the tumor’s large size, open thymectomy was performed instead of mini-invasive thoracoscopy, which is helpful to increase the R0 resection rate. Literature review has indicated that a male with a large anterior mediastinal mass should consider the possibility of thymic TAC. These patients might have concomitant diseases, such as Cushing syndrome or MEN type I syndrome. Radical resection is still the first choice for those early or locally advanced thymic TAC patients. The favorable prognosis can be achieved after radical surgery, and postoperative adjuvant therapies should be taken into consideration seriously.
However, there were several limitations in the whole course of treatment. First, neo-adjuvant chemotherapy or radiochemotherapy was not performed, which may induce tumor decreased and easier to remove. Second, the follow-up time is relatively short and a long-term follow-up is very necessary to evaluate the therapeutic efficacy.
In conclusion, for those who have a large mediastinal mass with compression syndrome, open surgery is a safe and effective method for locally advanced TAC and radical resection combination with adjuvant chemotherapy may lead to long-term survival.
Acknowledgments
We are thankful to Prof. Luying Tang and Dor. Jiexia Guan from the Institute of Pathology, the Third Affiliated Hospital of Sun Yat-Sen University, China, who provided the confirmation of the pathologic diagnosis of the patient.
Funding: None.
Footnote
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure from (available at http://dx.doi.org/10.21037/tcr.2020.02.10). The authors have no conflicts of interests to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Marx A, Chan JK, Coindre JM, et al. The 2015 World Health Organization Classification of Tumors of the Thymus: Continuity and Changes. J Thorac Oncol 2015;10:1383-95. [Crossref] [PubMed]
- Suster S, Moran CA. Neuroendocrine neoplasms of the mediastinum. Am J Clin Pathol 2001;115:S17-27. [PubMed]
- Sato H, Kajiya H, Kanai G, et al. Atypical thymic carcinoid associated with Cushing's syndrome. Tokai J Exp Clin Med 2010;35:78-84. [PubMed]
- Otake Y, Aoki M, Nakanishi T, et al. Atypical carcinoid of thymus associated with multiple endocrine neoplasia syndrome type 1. Gen Thorac Cardiovasc Surg 2010;58:534-7. [Crossref] [PubMed]
- Moran CA, Suster S. Neuroendocrine carcinomas (carcinoid tumor) of the thymus. A clinicopathologic analysis of 80 cases. Am J Clin Pathol 2000;114:100-10. [Crossref] [PubMed]
- Riley DS, Barber MS, Kienle GS, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol 2017;89:218-35. [Crossref] [PubMed]
- Ruffini E, Filosso PL, Mossetti C, et al. Thymoma: inter-relationships among World Health Organization histology, Masaoka staging and myasthenia gravis and their independent prognostic significance: a single-centre experience. Eur J Cardiothorac Surg 2011;40:146-53. [Crossref] [PubMed]
- Shi C, Klimstra DS. Pancreatic neuroendocrine tumors: pathologic and molecular characteristics. Semin Diagn Pathol 2014;31:498-511. [Crossref] [PubMed]
- Chejfec G, Falkmer S, Askensten U, et al. Neuroendocrine tumors of the gastrointestinal tract. Pathol Res Pract 1988;183:143-54. [Crossref] [PubMed]
- Caplin ME, Baudin E, Ferolla P, et al. Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann Oncol 2015;26:1604-20. [Crossref] [PubMed]
- McCormack A, Dekkers OM, Petersenn S, et al. Treatment of aggressive pituitary tumours and carcinomas: results of a European Society of Endocrinology (ESE) survey 2016. Eur J Endocrinol 2018;178:265-76. [Crossref] [PubMed]
- Frank-Raue K, Machens A, Leidig-Bruckner G, et al. Prevalence and clinical spectrum of nonsecretory medullary thyroid carcinoma in a series of 839 patients with sporadic medullary thyroid carcinoma. Thyroid 2013;23:294-300. [Crossref] [PubMed]
- Howitt BE, Kelly P, McCluggage WG. Pathology of Neuroendocrine Tumours of the Female Genital Tract. Curr Oncol Rep 2017;19:59. [Crossref] [PubMed]
- Rosai J, Higa E. Mediastinal endocrine neoplasm, of probable thymic origin, related to carcinoid tumor. Clinicopathologic study of 8 cases. Cancer 1972;29:1061-74. [Crossref] [PubMed]
- Gaur P, Leary C, Yao JC. Thymic neuroendocrine tumors: a SEER database analysis of 160 patients. Ann Surg 2010;251:1117-21. [Crossref] [PubMed]
- Crona J, Bjorklund P, Welin S, et al. Treatment, prognostic markers and survival in thymic neuroendocrine tumours. a study from a single tertiary referral centre. Lung Cancer 2013;79:289-93. [Crossref] [PubMed]
- Filosso PL, Yao X, Ahmad U, et al. Outcome of primary neuroendocrine tumors of the thymus: a joint analysis of the International Thymic Malignancy Interest Group and the European Society of Thoracic Surgeons databases. J Thorac Cardiovasc Surg 2015;149:103-9.e2. [Crossref] [PubMed]
- Szolkowska M, Szczepulska-Wojcik E, Maksymiuk B, et al. Primary mediastinal neoplasms: a report of 1,005 cases from a single institution. J Thorac Dis 2019;11:2498-511. [Crossref] [PubMed]
- Shimamoto A, Ashizawa K, Kido Y, et al. CT and MRI findings of thymic carcinoid. Br J Radiol 2017;90:20150341. [Crossref] [PubMed]
- Kan X, Wang P, Gong Z, et al. Investigation on Computed Tomography Features of Primary Thymic Atypical Carcinoid Tumors. J Comput Assist Tomogr 2017;41:990-4. [Crossref] [PubMed]
- Walts AE, Frye J, Engman DM, et al. Carcinoid tumors of the thymus and Cushing's syndrome: Clinicopathologic features and current best evidence regarding the cell of origin of these unusual neoplasms. Ann Diagn Pathol 2019;38:71-9. [Crossref] [PubMed]
- Meinardi JR, van den Berg G, Wolffenbuttel BH, et al. Cyclical Cushing's syndrome due to an atypical thymic carcinoid. Neth J Med 2006;64:23-7. [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Strobel P, Zettl A, Shilo K, et al. Tumor genetics and survival of thymic neuroendocrine neoplasms: a multi-institutional clinicopathologic study. Genes Chromosomes Cancer 2014;53:738-49. [Crossref] [PubMed]
- Ose N, Maeda H, Inoue M, et al. Results of treatment for thymic neuroendocrine tumours: multicentre clinicopathological study. Interact Cardiovasc Thorac Surg 2018;26:18-24. [Crossref] [PubMed]
- Araki T, Sholl LM, Hatabu H, et al. Radiological features and metastatic patterns of thymic neuroendocrine tumours. Clin Radiol 2018;73:479-84. [Crossref] [PubMed]
- Wu X, Qi Y, Yang F, et al. Spinal Metastasis Resulting from Atypical Thymic Carcinoid: A Case Report. World Neurosurg 2018;111:373-6. [Crossref] [PubMed]
- Sullivan JL, Weksler B. Neuroendocrine Tumors of the Thymus: Analysis of Factors Affecting Survival in 254 Patients. Ann Thorac Surg 2017;103:935-9. [Crossref] [PubMed]
- Cardillo G, Rea F, Lucchi M, et al. Primary neuroendocrine tumors of the thymus: a multicenter experience of 35 patients. Ann Thorac Surg 2012;94:241-5; discussion 245-6. [Crossref] [PubMed]
- Han B, Sun JM, Ahn JS, et al. Clinical outcomes of atypical carcinoid tumors of the lung and thymus: 7-year experience of a rare malignancy at single institute. Med Oncol 2013;30:479. [Crossref] [PubMed]