
The utility of histological subtype for predicting survival of lung cancer patients with rheumatoid arthritis
Introduction
Cancer and rheumatic disease sometimes coexist in the same patient, either simultaneously or sequentially. Although they can occur by accident, previous studies have reported that cancers may be closely linked to a state of continuous inflammation characterized by the production of cytokines, chemokines, and free radicals, thus contributing to an increased risk of carcinogenesis (1). Rheumatoid arthritis (RA) is a systemic autoimmune disease with chronic inflammation involving multiple organs. RA can sometimes affect the lung, as a frequent site of extra-articular involvement. Treatments for RA, including methotrexate and anti-tumor necrosis factor (TNF) antagonists, may increase the risk of malignancy (2-4). In epidemiological studies conducted in several countries, a positive association between increased incidence of lung cancer (LC) and RA has been reported (5-8).
The survival rate of LC patients with RA may be worse than that of those without RA, because the administration of long-term immunosuppressive therapies and impaired immune surveillance associated with underlying RA may promote cancer cell proliferation and survival (7,9). Underlying immune conditions in RA may also increase the risk of infection and treatment-related toxicity, a major cause of death during cancer treatment (10). However, periodic monitoring and longitudinal follow-ups of RA patients might result in better outcomes because of the increased likelihood of early detection and prompt treatment of LC (11). Although previous studies have suggested a significant link between RA and the incidence of LC, data regarding survival of LC patients, with or without RA, are limited and often conflicting (12-16). LC manifests in various histological subtypes, such as small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC), so it is necessary to assess the outcome measures histologically in LC patients with RA. However, previous studies that reported the outcomes of LC patients with RA did not include information about the histological subtypes. Thus, it is unknown whether there was a significant difference in survival of LC patients with RA by histological subtypes. The aim of this study was therefore to assess whether LC patients with RA, treated in a single tertiary university-affiliated hospital in South Korea, had worse outcomes than those without RA, by separately analyzing the results of SCLC and NSCLC patients, and identify potential factors, including the presence of RA, which might predict outcomes.
Methods
Study population and data collection
The medical records of patients with RA and concurrent LC diagnosed at Hanyang University Hospital, a tertiary referral hospital in South Korea, between January 1, 2011 and December 31, 2016, were retrospectively analyzed. We only included LC patients who received treatment for LC; we excluded patients with cancer diagnosed before RA diagnosis, because the aim of the study was to investigate the possible effect of RA on the survival of LC patients. Patients with other rheumatic diseases, except RA, HIV infections, or other active malignancies, were also excluded.
We identified 46 LC patients with rheumatic diseases, among whom 9 with other rheumatic diseases were excluded (4 with systemic sclerosis, 3 with systemic lupus erythematosus, 1 with overlap syndrome, and 1 with Behçet’s disease). Three LC patients with RA were not treated. A total of 34 LC patients with RA were treated at our hospital. For comparative purposes, patients with newly diagnosed LC treated in our respiratory clinic during the same 6-year study period, without any evidence of systemic rheumatic diseases including RA, were matched and served as the control group. Four controls were randomly selected from among the LC patients without RA and matched to one patient with RA by age and sex. This ratio of 1:4 was used throughout, except in two RA cases with a ratio of 1:2. Finally, 132 age- and sex-matched patients without RA who received medical care at the same hospital were included in this study.
Data collection
The diagnosis of RA was established by a board certified rheumatologist, according to the 1987 American College of Rheumatology (formerly the American Rheumatism Association) revised classification criteria for RA (17) and the 2010 American College of Rheumatology /European League against Rheumatism criteria (18). The diagnosis of LC was confirmed by cytological or histological examination. Data on numerous factors associated with mortality were collected for the LC patients, including sex, body mass index (BMI), Charlson comorbidity index (CCI), smoking history, age at time of diagnosis of LC and RA, calendar year of LC diagnosis, tumor histology, cancer stage, genetic mutation status, underlying lung disease status, and treatments for LC and RA. We checked for the presence of interstitial lung disease (ILD) using chest computed tomography (CT), and for chronic lung diseases including asthma and chronic obstructive pulmonary disease (COPD). The CCI score, as a measure of comorbidity, encompasses 22 comorbid conditions, including cardiovascular disease, malignancy, and lung disease (19). The scores for connective tissue disease and LC were omitted from the CCI score in this study. The cancer stage was determined based on the international tumor-node-metastasis (TNM) criteria. Mutational analysis was performed on DNA extracted from surgically resected, biopsied, or cytological specimens of NSCLC patients. Epidermal growth factor receptor (EGFR) mutational status was tested using quantitative real-time polymerase chain reaction (qPCR) with the PNAClamp EGFR Mutation Detection Kit (Panagene, Daejeon, South Korea).
Statistical analysis
We used SAS software (version 9.4; SAS Institute, Cary, NC, USA) for matching the patients, and R software (version 3.4.2; R Development Core Team, Vienna, Austria) for all other statistical analyses (after first assessing the normality of the data distribution using the Shapiro-Wilk test). Patient matching based on age and sex data extracted from medical records was conducted using the Greedy algorithm. We compared clinicopathological characteristics between LC patients with and without RA. Continuous variables were compared using the t-test or Mann-Whitney U test, and categorical variables using the χ2 or Fisher’s exact tests. All nominal variables are expressed as frequencies and percentages. Continuous variables are expressed as means ± SD if normally distributed, while non-normally distributed variables are expressed as medians with quartile range. Follow-up began at the time of cancer diagnosis and ended at the time of death or the last follow-up visit for patients without an event (whichever occurred first).
Survival curves were estimated using the Kaplan-Meier method, and differences in survival among groups were assessed using a two-sided log-rank test. We analyzed the univariate associations of mortality with clinicopathological variables of interest using a Cox proportional hazards regression model, and hazard ratios (HRs) and P values were calculated. Next, we applied the best subset selection method to build multivariate models including the variables that were significant in the univariate analysis, using the backward stepwise selection method. We computed the Akaike information criterion (AIC) for a set of candidate models with different numbers of variables and selected the one with the smallest AIC value. In the same manner, we also separately constructed best-fit models based on NSCLC and SCLC histologies. In addition, lung adenocarcinoma and squamous cell carcinoma represent the major subtypes in NSCLC, and these histologic features might be important to assess the outcome of NSCLC patients. Thus, an analysis was conducted on the impact of RA presence on mortality in two main histological types of NSCLC (lung adenocarcinoma and squamous cell carcinoma). All tests were two-sided, and a P value <0.05 was considered to reflect statistical significance.
Ethics statement
The study protocol was approved by the Institutional Review Board (IRB) of Hanyang University Hospital, Seoul, South Korea (IRB No. 2019-06-016). All data were anonymized before the analysis, and the requirement for informed consent from the study participants was waived because of the retrospective nature of the study.
Results
Baseline characteristics of RA patients with LC
The baseline characteristics of the 166 LC patients enrolled in this study are shown in Table 1. Of the 34 LC patients with RA, 55.9% were male, and 61.8% were active or former smokers; these proportions showed no statistical difference from the group of LC patients without RA. Lung adenocarcinoma predominated in both groups; however, a significant difference was found in the frequency thereof between the groups; adenocarcinoma was diagnosed in 35.5% of the RA patients, which was a lower proportion than in the LC patients without RA (60.6%; P=0.0317). A larger proportion of SCLC was noted in LC patients with versus without RA (26.5% vs. 12.9%). LC patients with RA had a significantly lower BMI than those without RA (P=0.0488). Although more LC patients with RA had a CCI ≥2 than those without RA, the difference was not significant (35.3% vs. 18.9%; P=0.0596). ILD was significantly more prevalent in LC patients with RA than in those without (26.5% vs. 1.5%; P<0.0001), whereas no significant between-group difference was noted in the frequency of chronic lung diseases, such as asthma or COPD. No group difference was seen in age at LC diagnosis, calendar year of LC diagnosis, TNM stage, EGFR mutant status in NSCLC, or anti-cancer treatment status. The proportion of extensive stage disease of SCLC, and of stage IV NSCLC, did not differ between the two groups. The LC treatment status was similar between the two groups. In particular, the rates of chemotherapy alone (38.2%) and combined therapy (23.5%) in LC patients with RA were comparable to those of patients without RA. Similar to the result in all LC patients, NSCLC with RA had lower BMI and higher prevalence of ILD, compared to those without RA. No significant between-group difference was noted in TNM stage, EGFR mutation status, and anti-cancer treatment (Table S1). Only two LC patients without RA (one with adenocarcinoma and one with squamous cell carcinoma) were treated with immunotherapy, and none of the LC patients with RA were treated with immunotherapy. Among LC patients with RA, no difference was noted between the SCLC and NSCLC patients in the mean age at RA diagnosis, duration of RA before LC diagnosis, or RA treatment status. Three NSCLC patients with RA were treated with an anti-TNF antagonist (Table S2).
Table 1
| Variables | Lung cancer with RA (N=34), n (%) | Lung cancer without RA (N=132), n (%) | P value |
|---|---|---|---|
| Gender | 1.0000 | ||
| Female | 15 (44.1) | 56 (42.4) | |
| Male | 19 (55.9) | 76 (57.6) | |
| Body mass index | 22.37±3.25 | 23.80±3.73 | 0.0488 |
| Age at cancer diagnosis | 67.12±8.35 | 66.58±7.89 | 0.7760 |
| Calendar year at cancer diagnosis | 0.7267 | ||
| 2011–2012 | 13 (38.2) | 42 (31.8) | |
| 2013–2014 | 11 (32.4) | 43 (32.6) | |
| 2015–2016 | 10 (29.4) | 47 (35.6) | |
| Charlson Comorbidity Index | 0.0596 | ||
| 0 | 9 (26.5) | 60 (45.5) | |
| 1 | 13 (38.2) | 47 (35.6) | |
| ≥2 | 12 (35.3) | 25 (18.9) | |
| Smoking, ever | 21 (61.8) | 72 (54.5) | 0.5738 |
| Cancer type | 0.0317 | ||
| Small cell lung cancer | 9 (26.5) | 17 (12.9) | |
| Adenocarcinoma | 12 (35.3) | 80 (60.6) | |
| Squamous | 11 (32.4) | 26 (19.7) | |
| Others | 2 (5.9) | 9 (6.8) | |
| Stage | 0.4089 | ||
| I–III | 20 (58.8) | 90 (68.2) | |
| IV | 14 (41.2) | 42 (31.8) | |
| Stage (each cancer cell type) | |||
| Small cell lung cancer | 0.6828 | ||
| Limited stage disease | 3 (33.3) | 8 (47.1) | |
| Extensive stage disease | 6 (66.7) | 9 (52.9) | |
| Non-small cell lung cancer | 0.3421 | ||
| I | 14 (56.0) | 53 (46.1) | |
| II | 2 (8.0) | 8 (7.0) | |
| III | 1 (4.0) | 21 (18.3) | |
| IV | 8 (32.0) | 33 (28.7) | |
| Underlying lung disease | |||
| ILD | 9 (26.5) | 2 (1.5) | <0.0001 |
| Chronic lung diseases, other than ILD* | 9 (26.5) | 26 (19.7) | 0.5302 |
| EGFR mutant** | 5 (31.2) | 25 (32.5) | 1.0000 |
| Treatment | |||
| Surgery, only | 10 (29.4) | 41 (31.1) | 1.0000 |
| Chemotherapy, only | 13 (38.2) | 45 (34.1) | 0.8024 |
| Radiation, only | 3 (8.8) | 4 (3.0) | 0.1522 |
| 2 or more treatments | 8 (23.5) | 42 (31.8) | 0.4655 |
| Survival | 0.5766 | ||
| Alive | 12 (35.3) | 56 (42.4) | |
| Death | 22 (64.7) | 76 (57.6) |
*Chronic lung diseases, other than ILD included asthma and chronic obstructive pulmonary diseases; **EGFR mutation test was performed in 16 patients with RA and 77 patients without RA. ILD, interstitial lung disease; RA, rheumatoid arthritis.
Overall survival by RA status
During the study period, 22 (64.7%) LC patients with RA and 76 (57.6%) LC patients without RA died (Table 1). The overall survival rate did not differ between LC patients with RA and age- and sex-matched LC patients without RA (Figure 1A), even when the survival analysis was conducted on SCLC and NSCLC patient subgroups (Figure 1B,C). Risk factors predictive of overall mortality based on multivariate Cox model analysis are listed in Table 2. Among LC patients of all histological subtypes, low BMI, older age at LC diagnosis, high CCI score, small cell histology, and advanced stage IV disease were significant risk factors for mortality. The status of RA was not a significant predictor of mortality after adjusting for other variables. SCLC is a more aggressive subtype of LC than NSCLC (20). As expected, mortality was significantly worse in RA patients with small cell histology compared to those with NSCLC (P=0.0404, Figure 2). Risk factor analysis was therefore conducted regarding mortality in LC patients with RA according to histological subtype (SCLC vs. NSCLC). We found that RA (HR: 3.26; P=0.0350) was a poor prognostic indicator for SCLC patients, along with high BMI (HR: 1.26, P=0.0275) and stage IV disease (HR: 5.28; P=0.0033). However, RA did not influence the mortality risk in NSCLC patients. Low BMI, high CCI score, smoking, and advanced stage IV were significant risk factors for mortality in NSCLC patients. Table 3 represents results from univariate and multivariate analysis of mortality according to the two main histological types of NSCLC (lung adenocarcinoma and squamous cell carcinoma). There were 92 (55.4% of total) patients for lung adenocarcinoma and 37 (22.3%) for squamous cell lung cancer. Lung adenocarcinoma histology was a more favorable risk factor predictive of mortality in NSCLC patients, compared to squamous cell carcinoma, but there was no statistical significance (HR: 0.53, P=0.0565). In multivariate analysis of patients with lung adenocarcinoma, male gender (HR: 0.25, P=0.0052), BMI (HR: 0.83, P=0.0002), ever smoking (HR: 4.20, P=0.0035), and advanced stage IV disease (HR: 2.76, P=0.0094) were significant factors associated with mortality. Among those with lung squamous cell carcinoma, only advanced stage IV disease was a significant factor for mortality. This study, however, the presence of RA had no significant impact on the mortality even in two subgroups with lung adenocarcinoma and squamous cell carcinoma.

Table 2
| Variables | Mortality in all lung cancer | Mortality in SCLC | Mortality in NSCLC | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | Univariable | Multivariable | ||||||||||||
| HR | P value | HR | P value | HR | P value | HR | P value | HR | P value | HR | P value | ||||||
| RA (vs. non-RA) | 1.36 | 0.2098 | 1.53 | 0.3661 | 3.26 | 0.0350 | 1.18 | 0.5789 | |||||||||
| Male gender (vs. female) | 1.72 | 0.0114 | 0.58 | 0.2348 | 1.96 | 0.0051 | |||||||||||
| Body mass index (n=165) | 0.93 | 0.0157 | 0.91 | 0.0088 | 1.19 | 0.0315 | 1.26 | 0.0275 | 0.89 | 0.0007 | 0.89 | 0.0019 | |||||
| Age at cancer diagnosis | 1.04 | 0.0078 | 1.03 | 0.0241 | 1.06 | 0.0312 | 1.04 | 0.1620 | 1.03 | 0.0558 | 1.03 | 0.0942 | |||||
| CCI score | |||||||||||||||||
| CCI = 1 (vs. 0) | 1.78 | 0.0145 | 1.71 | 0.0313 | 1.01 | 0.9873 | 1.88 | 0.0163 | 2.02 | 0.0208 | |||||||
| CCI ≥ 2 (vs. 0) | 1.70 | 0.0496 | 1.79 | 0.0420 | 1.70 | 0.3410 | 1.63 | 0.1146 | 1.96 | 0.0496 | |||||||
| Smoking, ever | 2.42 | 0.0001 | 1.63 | 0.0578 | 0.48 | 0.1590 | 2.61 | 0.0001 | 2.15 | 0.0063 | |||||||
| SCLC (vs. NSCLC) | 2.63 | 0.0001 | 1.75 | 0.0439 | NA | NA | |||||||||||
| Stage IV (vs. I–III) | 5.80 | <0.0001 | 4.24 | <0.0001 | 5.39 | 0.0015 | 5.28 | 0.0033 | 5.33 | <0.0001 | 3.52 | <0.0001 | |||||
| Underlying lung disease | |||||||||||||||||
| ILD | 1.65 | 0.1776 | 0.93 | 0.8845 | 1.21 | 0.7533 | |||||||||||
| Chronic lung disease, other than ILD | 1.38 | 0.1703 | 3.19 | 0.0493 | 1.37 | 0.2336 | 0.55 | 0.0665 | |||||||||
| Treatment | |||||||||||||||||
| Surgery only | 0.17 | <0.0001 | 0.47 | 0.0505 | NA | 0.19 | <0.0001 | 0.44 | 0.0346 | ||||||||
| Chemotherapy only | 3.54 | <0.0001 | 1.56 | 0.1209 | 2.34 | 0.0687 | 3.41 | <0.0001 | 1.74 | 0.1145 | |||||||
| Radiation only | 3.92 | 0.0015 | 3.74 | 0.0086 | 11.99 | 0.0426 | 4.10 | 0.0029 | 4.14 | 0.0113 | |||||||
| 2 or more treatments | 0.93 | 0.7550 | 0.35 | 0.0300 | 1.08 | 0.7494 | |||||||||||
CCI, Charlson Comorbidity Index; HR, hazard ratios; ILD, interstitial lung disease; NA, not applicable; NSCLC, non-small cell lung cancer; RA, rheumatoid arthritis; SCLC, small cell lung cancer.
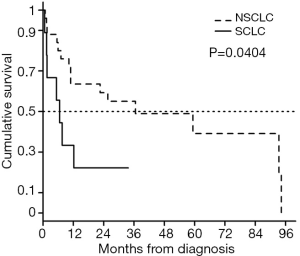
Table 3
| Variables | Mortality in lung adenocarcinoma and squamous cell carcinoma (N=129) | Mortality in lung adenocarcinoma (N=92) | Mortality in lung squamous cell carcinoma (N=37) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | Univariable | Multivariable | ||||||||||||
| HR | P value | HR | P value | HR | P value | HR | P value | HR | P value | HR | P value | ||||||
| RA (vs. non-RA) | 1.09 | 0.7883 | 0.58 | 0.1294 | 1.44 | 0.4095 | 0.42 | 0.1024 | 0.45 | 0.1447 | |||||||
| Male gender (vs. female) | 1.88 | 0.0127 | 1.73 | 0.0738 | 1.29 | 0.6476 | |||||||||||
| Body mass index (n=165) | 0.91 | 0.0060 | 0.92 | 0.0385 | 0.88 | 0.0030 | 0.85 | 0.0018 | 1.03 | 0.6787 | |||||||
| Age at cancer diagnosis | 1.04 | 0.0319 | 1.05 | 0.0096 | 1.04 | 0.0614 | 1.05 | 0.0188 | 1.03 | 0.3612 | |||||||
| CCI score | |||||||||||||||||
| CCI = 1 (vs. 0) | 1.87 | 0.0253 | 1.66 | 0.0841 | 2.33 | 0.0135 | 1.27 | 0.6426 | |||||||||
| CCI ≥ 2 (vs. 0) | 1.93 | 0.0442 | 2.28 | 0.0163 | 1.61 | 0.3009 | 2.08 | 0.1550 | |||||||||
| Smoking, ever | 2.65 | 0.0002 | 1.91 | 0.0411 | 2.72 | 0.0011 | 1.96 | 0.3646 | |||||||||
| Adenoca (vs. Squamous) | 0.60 | 0.0420 | 0.53 | 0.0565 | NA | NA | |||||||||||
| Stage IV (vs. I–III) | 4.91 | <0.0001 | 6.97 | <0.0001 | 6.36 | <0.0001 | 3.17 | 0.0009 | 10.63 | 0.0001 | 15.21 | <0.0001 | |||||
| Underlying lung disease | |||||||||||||||||
| ILD | 0.89 | 0.8689 | 10.87 | 0.0245 | 0.26 | 0.2012 | 0.17 | 0.1521 | |||||||||
| Chronic lung disease, other than ILD | 1.46 | 0.1756 | 1.75 | 0.1115 | 1.06 | 0.8969 | 2.67 | 0.0642 | |||||||||
| Treatment | |||||||||||||||||
| Surgery only | 0.19 | <0.0001 | 0.05 | <0.0001 | 0.07 | 0.0006 | 0.65 | 0.3577 | |||||||||
| Chemotherapy only | 3.54 | <0.0001 | 4.45 | <0.0001 | 5.14 | 0.0016 | |||||||||||
| Radiation only | 3.72 | 0.0288 | 12.59 | 0.0013 | 4.89 | 0.0575 | 1.11 | 0.9181 | |||||||||
| 2 or more treatments | 1.14 | 0.6075 | 1.43 | 0.2858 | 0.67 | 0.3450 | |||||||||||
CCI, Charlson Comorbidity Index; HR, hazard ratios; ILD, interstitial lung disease; NA, not applicable; NSCLC, non-small cell lung cancer; RA, rheumatoid arthritis; SCLC, small cell lung cancer.
Discussion
In this retrospective cohort study of Korean RA patients, we showed that RA did not reduce overall survival in LC patients. No difference in overall survival was found in survival analysis stratified by small cell versus non-small cell histology. However, RA patients with SCLC had significantly worse outcomes than those with NSCLC. We also found that the presence of RA was a significant predictor of mortality among SCLC patients, along with BMI and LC stage status, whereas RA among NSCLC patients was not associated with an increased mortality risk.
In an analysis of Korean patients with all types of rheumatic diseases, RA was associated with higher mortality in LC patients after adjusting for other variables (15). In contrast, a Chinese study reported that RA did not shorten the survival of LC patients with RA compared to the general population (21). Ji et al. (13) analyzed Swedish hospital discharge register data and reported no significant difference in overall survival between LC patients with versus without RA. However, these conflicting results regarding the effect of RA on the survival of LC patients might be related to heterogeneity in the definitions of LC. Small cell and non-small cell are the two main subtypes of LC, which have several key differences in terms of treatment response and prognosis (22). Most previous studies considered LC to be a single entity, with no distinction made between SCLC and NSCLC (13,15,21,23). Only a few studies have assessed survival rates in LC patients with RA according to histological subtype. Abasolo et al. (12) evaluated survival rates in Spanish RA patients of all subtypes, and reported that mortality was significantly higher than expected for lung adenocarcinoma in males, while in a Swedish national cancer registry-based study, Hemminki et al. (14) found a potential association of LC mortality with all types of autoimmune diseases, including RA, but failed to find a significantly increased HR for mortality in an analysis of the four main histological subtypes (adenocarcinoma, squamous, large cell, and small cell). Our study differed in several respects from the above two epidemiological studies, which evaluated LC survival in RA via subgroup analysis. To the best of our knowledge, our study is the first cohort study to analyze survival rates by histological subtype of LC, including SCLC and NSCLC, and to identify potential factors predicting the mortality of LC patients.
Our analyses revealed that RA was not associated with increased mortality in LC patients, irrespective of histological subtype. Cancer survival depends mainly on sex, age, cancer stage, smoking status, and comorbidities, all of which affect treatment outcomes. As reported in a previous epidemiological study, LC predominantly affected males (24). In contrast, Chen et al. (21) and Liu et al. (25) conducted cohort studies comparing LC patients with RA to those without RA, and reported that female patients accounted for nearly half of the LC patients with RA. Similarly, in our study 44.1% of the LC patients with RA were females, which might be attributed to the high prevalence of RA in females (26). Because sex differences in LC survival have been reported (27), we used a sex- and age-matched control group in the present study. The smoking rates were similar between the groups. There was no significant difference in CCI or TNM stage between LC patients with RA and age- and sex-matched controls, so these factors would not have affected the results regarding morality. However, ILD was observed in 26.5% of LC patients with RA, which was a much higher rate than in those without RA, despite the similar proportion of smokers between the groups. In a previous report (25), 40.9% of LC patients with RA had ILD, which was consistent with our findings. ILD is a well-known and frequently reported extraarticular manifestation of RA (28), and could negatively affect the survival of RA patients (29). Alternatively, LC in RA-associated ILD might be hidden until it manifests clinically, thus resulting in delayed detection (30), while ILD-associated accumulation of autoimmune cells in RA patients could create a harsh environment preventing cancer cell growth (31). We found that the presence of ILD was not significantly associated with a worse outcome in LC patients in a multivariable Cox regression analysis controlling other variables. One possible reason for these findings was that respiratory symptoms associated with LC, but not ILD, may facilitate diagnostic imaging for early detection of ILD or very mild ILD, which would otherwise be asymptomatic. Nonetheless, the impact of ILD on survival in LC patients with RA still needs further study.
Our analyses based on LC subtype revealed that RA was significantly associated with the lower survival rate of SCLC, but not NSCLC, patients. We also found that consistent results that RA had no large influence on the survival of NSCLC patients, irrespective of histological types including lung adenocarcinoma and squamous cell histology. Several potential mechanisms may explain the association of RA with the risk of mortality in SCLC patients, including the poor prognosis of SCLC. Because the main treatment for SCLC is chemotherapy, compared to surgery for NSCLC, it is possible that RA and its related comorbidities might be more treatment-limiting factors when the duration of treatment is extended. Second, the outcome of interest in the present study was all-cause mortality; the mortality of LC patients with RA may be affected by various factors, including cancer stage, comorbid conditions and treatment, and the effects of the RA itself. All of these factors could cause an increase in mortality. It is possible that the mortality of cases of LC discovered at an early stage may not be associated with LC itself, but rather with cardiovascular diseases and other non-RA-related causes. We found that 64% of NSCLC patients with RA were of stage I–II, which was a higher proportion versus those without RA (53.1%), although not significantly. However, two-thirds of SCLC patients with RA had extensive stage disease. Thus, the effects of RA on LC mortality could differ between SCLC and NSCLC. These intriguing findings provide the basis for future investigations.
Due to the regular follow-up of RA patients, we expected that earlier stage cancers would be detected (11). Surprisingly, however, the majority of SCLC cases were detected at an advanced stage in the LC with RA group. Because RA patients with advanced LC are more likely to be referred to our center, a selection bias may have existed. However, early detection of SCLC using imaging alone is challenging because SCLC can metastasize even when the primary lesion is very small. Our findings raise concerns regarding potential negligence of SCLC in RA patients attending regular follow-ups, and highlight the need for optimization of the LC screening program for RA patients. Further studies are also needed to compare cancer screening rates between patients with RA and the general population.
Our study had several limitations. First, it was performed at a single institution and included a relatively small sample size, so there may have been a bias in patient selection. Previous studies have described a few cases of SCLC in LC patients with RA, predominantly as adenocarcinomas (21,25); however, in this study, a significantly higher proportion of LC patients with RA had SCLC versus those without RA, which may have affected our results. Second, the use of LC immunotherapy has become clinically important in the treatment of LC patients with rheumatic diseases (32), including RA. In the present study, only two LC patients were treated with immunotherapy without RA. Thus, our findings cannot be generalized to other populations treated with immunotherapy. Third, we excluded patients who were untreated. Because LC patients with RA have been reported to be less likely to receive anti-cancer treatment (25), our results may not be fully applicable to clinical practice. Finally, our study was limited by its retrospective nature; we had information only on all-cause mortality, and not on LC-specific mortality. Thus, our survival data should be interpreted with caution.
Conclusions
No significant difference in survival was found between LC patients with versus without RA, including in subgroup analysis of SCLC and NSCLC. Notably, regarding the association of RA with poor outcomes in LC patients, the presence of RA could be a significant factor impairing overall survival in SCLC, but not NSCLC, patients. Future studies assessing the relevance of RA to the prognosis of LC patients should recognize that the histological subtype may affect the outcome.
Table S1
| Variables | Lung cancer with RA (N=25) | Lung cancer without RA (N=115) | P value |
|---|---|---|---|
| Gender | 0.8490 | ||
| Female | 12 (48.0) | 50 (43.5) | |
| Male | 13 (52.0) | 65 (56.5) | |
| BMI | 21.86±3.42 | 23.69±3.85 | 0.0275 |
| Age at cancer diagnosis | 66.80±7.84 | 66.38±7.66 | 0.8062 |
| Calendar year at cancer diagnosis | 0.5734 | ||
| 2011–2012 | 11 (44.0) | 38 (33.0) | |
| 2013–2014 | 6 (24.0) | 35 (30.4) | |
| 2015–2016 | 8 (32.0) | 42 (36.5) | |
| Charlson Comorbidity Index | 0.2508 | ||
| 0 | 7 (28.0) | 53 (46.1) | |
| 1 | 11 (44.0) | 39 (33.9) | |
| ≥2 | 7 (28.0) | 23 (20.0) | |
| Smoking, ever | 15 (60.0) | 58 (50.4) | 0.5177 |
| Cancer type | 0.0763 | ||
| Adenocarcinoma | 12 (48.0) | 80 (69.6) | |
| Squamous | 11 (44.0) | 26 (22.6) | |
| Others | 2 (8.0) | 9 (7.8) | |
| Stage | 0.3421 | ||
| I | 14 (56.0) | 53 (46.1) | |
| II | 2 (8.0) | 8 (7.0) | |
| III | 1 (4.0) | 21 (18.3) | |
| IV | 8 (32.0) | 33 (28.7) | |
| Underlying lung disease | |||
| ILD | 4 (16.0) | 0 (0.0) | 0.0008 |
| Chronic lung diseases, other than ILD* | 6 (24.0) | 25 (21.7) | 1.0000 |
| EGFR mutant** | 5 (31.2) | 25 (32.5) | 1.0000 |
| Treatment | |||
| Surgery, only | 10 (40.0) | 41 (35.7) | 0.8570 |
| Chemotherapy, only | 7 (28.0) | 36 (31.3) | 0.9319 |
| Radiation, only | 2 (8.0) | 4 (3.5) | 0.2911 |
| 2 or more treatments | 6 (24.0) | 34 (29.6) | 0.7535 |
| Survival | 0.7394 | ||
| Alive | 10 (40.0) | 53 (46.1) | |
| Death | 15 (60.0) | 62 (53.9) |
*Chronic lung diseases, other than ILD included asthma and chronic obstructive pulmonary diseases; **EGFR mutation test was performed in 16 patients with RA and 77 patients without RA. ILD, interstitial lung disease; RA, rheumatoid arthritis
Table S2
| Variables | SCLC with RA (N=9) | NSCLC with RA (N=25) | P value |
|---|---|---|---|
| Age of RA onset (year) | 60.44±9.51 | 56.88±11.17 | 0.4041 |
| RA duration before cancer diagnosis, median, (quartiles), months | 53.3 (22.1–144.7) | 82.7 (29.3–184.6) | 0.4465 |
| Used drugs for RA | |||
| MTX | 5 (55.6) | 14 (56.0) | 1.000 |
| Steroid | 6 (66.7) | 20 (80.0) | 0.6488 |
| Other DMARDs | 7 (77.8) | 17 (68.0) | 0.6921 |
| TNF inhibitors | 0 (0.0) | 3 (12.0) | 0.5488 |
| Others* | 1 (11.1) | 2 (8.0) | 1.000 |
*Others included tocilizumab, rituximab, abatacept, bucillamine, and cyclosporine. DMARDs, disease-modifying anti-rheumatic drugs; MTX, methotrexate; NSCLC, non-small cell lung cancer; RA, rheumatoid arthritis; SCLC, small cell lung cancer; TNF, tumor necrosis factor.
Acknowledgments
Funding: This research was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.03.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was approved by the Institutional Review Board (IRB) of Hanyang University Hospital, Seoul, South Korea (IRB No. 2019-06-016). All data were anonymized before the analysis, and the requirement for informed consent from the study participants was waived because of the retrospective nature of the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Franks AL, Slansky JE. Multiple associations between a broad spectrum of autoimmune diseases, chronic inflammatory diseases and cancer. Anticancer Res 2012;32:1119-36. [PubMed]
- Solomon DH, Kremer JM, Fisher M, et al. Comparative cancer risk associated with methotrexate, other non-biologic and biologic disease-modifying anti-rheumatic drugs. Semin Arthritis Rheum 2014;43:489-97. [Crossref] [PubMed]
- Bongartz T, Sutton AJ, Sweeting MJ, et al. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA 2006;295:2275-85. [Crossref] [PubMed]
- Jung SM, Kwok SK, Ju JH, et al. Risk of malignancy in patients with rheumatoid arthritis after anti-tumor necrosis factor therapy: results from Korean National Health Insurance claims data. Korean J Intern Med 2019;34:669-77. [Crossref] [PubMed]
- Chen YJ, Chang YT, Wang CB, et al. The risk of cancer in patients with rheumatoid arthritis: a nationwide cohort study in Taiwan. Arthritis Rheum 2011;63:352-8. [Crossref] [PubMed]
- Mercer LK, Davies R, Galloway JB, et al. Risk of cancer in patients receiving non-biologic disease-modifying therapy for rheumatoid arthritis compared with the UK general population. Rheumatology (Oxford) 2013;52:91-8. [Crossref] [PubMed]
- Turesson C, Matteson EL. Malignancy as a comorbidity in rheumatic diseases. Rheumatology (Oxford) 2013;52:5-14. [Crossref] [PubMed]
- Lee H. The Risk of Malignancy in Korean Patients with Rheumatoid Arthritis. Yonsei Med J 2019;60:223-9. [Crossref] [PubMed]
- Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest 2007;117:1137-46. [Crossref] [PubMed]
- Jayashree S, Nirekshana K, Guha G, et al. Cancer chemotherapeutics in rheumatoid arthritis: A convoluted connection. Biomed Pharmacother 2018;102:894-911. [Crossref] [PubMed]
- Hemminki K, Liu X, Ji J, et al. Effect of autoimmune diseases on mortality and survival in subsequent digestive tract cancers. Ann Oncol 2012;23:2179-84. [Crossref] [PubMed]
- Abasolo L, Judez E, Descalzo MA, et al. Cancer in rheumatoid arthritis: occurrence, mortality, and associated factors in a South European population. Semin Arthritis Rheum 2008;37:388-97. [Crossref] [PubMed]
- Ji J, Liu X, Sundquist K, et al. Survival of cancer in patients with rheumatoid arthritis: a follow-up study in Sweden of patients hospitalized with rheumatoid arthritis 1 year before diagnosis of cancer. Rheumatology (Oxford) 2011;50:1513-8. [Crossref] [PubMed]
- Hemminki K, Liu X, Ji J, et al. Effect of autoimmune diseases on risk and survival in histology-specific lung cancer. Eur Respir J 2012;40:1489-95. [Crossref] [PubMed]
- Park JK, Yang JA, Ahn EY, et al. Survival rates of cancer patients with and without rheumatic disease: a retrospective cohort analysis. BMC Cancer 2016;16:381. [Crossref] [PubMed]
- Nayak P, Luo R, Elting L, et al. Impact of Rheumatoid Arthritis on the Mortality of Elderly Patients Who Develop Cancer: A Population-Based Study. Arthritis Care Res (Hoboken) 2017;69:75-83. [Crossref] [PubMed]
- Silman AJ. The 1987 revised American Rheumatism Association criteria for rheumatoid arthritis. Br J Rheumatol 1988;27:341-3. [Crossref] [PubMed]
- Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569-81. [Crossref] [PubMed]
- Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011;173:676-82. [Crossref] [PubMed]
- Rodriguez E, Lilenbaum RC. Small cell lung cancer: past, present, and future. Curr Oncol Rep 2010;12:327-34. [Crossref] [PubMed]
- Chen CY, Chen YM, Yen SH, et al. Lung cancer associated with rheumatoid arthritis does not shorten life expectancy. J Chin Med Assoc 2005;68:216-20. [Crossref] [PubMed]
- Savas P, Hughes B, Solomon B. Targeted therapy in lung cancer: IPASS and beyond, keeping abreast of the explosion of targeted therapies for lung cancer. J Thorac Dis 2013;5:S579-92. [PubMed]
- Khan SA, Pruitt SL, Xuan L, et al. How does autoimmune disease impact treatment and outcomes among patients with lung cancer? A national SEER-Medicare analysis. Lung Cancer 2018;115:97-102. [Crossref] [PubMed]
- Jung KW, Won YJ, Kong HJ, et al. Survival of korean adult cancer patients by stage at diagnosis, 2006-2010: national cancer registry study. Cancer Res Treat 2013;45:162-71. [Crossref] [PubMed]
- Liu X, Xu Y, Zhou Q, et al. Clinicopathological features of lung cancer in patients with rheumatoid arthritis. J Thorac Dis 2018;10:3965-72. [Crossref] [PubMed]
- van Vollenhoven RF. Sex differences in rheumatoid arthritis: more than meets the eye. BMC Med 2009;7:12. [Crossref] [PubMed]
- Sagerup CM, Smastuen M, Johannesen TB, et al. Sex-specific trends in lung cancer incidence and survival: a population study of 40,118 cases. Thorax 2011;66:301-7. [Crossref] [PubMed]
- Yunt ZX, Solomon JJ. Lung disease in rheumatoid arthritis. Rheum Dis Clin North Am 2015;41:225-36. [Crossref] [PubMed]
- Kim YJ, Shim JS, Choi CB, et al. Mortality and incidence of malignancy in Korean patients with rheumatoid arthritis. J Rheumatol 2012;39:226-32. [Crossref] [PubMed]
- Wells AU, Denton CP. Interstitial lung disease in connective tissue disease--mechanisms and management. Nat Rev Rheumatol 2014;10:728-39. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Rangachari D, Costa DB. Moving the mountain in advanced non-small-cell lung cancer: evolving immunotherapies for a dire disease. Transl Cancer Res 2017;6:S151-7. [Crossref] [PubMed]


