A retrospective study comparing D1 limited lymph node dissection and D2 extended lymph node dissection for N3 gastric cancer
Introduction
Gastric cancer ranks globally as the second-highest contributor to cancer-related mortality, although its prevalence has been reducing (1). Eastern Asia accounts for around 6 in 10 newly diagnosed cases of gastric cancer, and more than 2 million new cases are diagnosed annually in China alone. A multidisciplinary approach has been proven to improve the outcome for advanced-stage gastric cancer patients, but surgical resection offers the most efficacy as a therapy for those with curable cases of the disease. Current data from level 1 evidence are based on N1 or N2 disease, and reports on N3 disease are limited (2-4).
The therapeutic value of limited (D1) vs. extended (D2) lymph node dissection for locally advanced gastric cancer remains controversial. The overall survival (OS) rate for N3 disease remains poor with radical surgery, including in Asian countries such as China, where extended lymph node dissection is practiced routinely. N3 disease was reported to have no survival benefits from any extensive surgery beyond D2, such as extensive para-aortic node dissection (D3) (5,6).
Whether D2 provides any survival benefits in the treatment of N3 disease remains unclear. This retrospective study compares two types of surgical management for lymph nodes of patients with gastric cancer, namely, D1 and D2 node dissection.
Methods
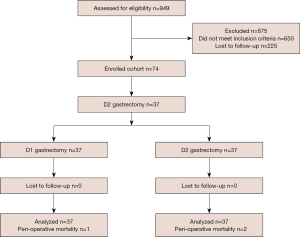
Among those who underwent R0 gastric resection from July 2010 to June 2015 in the 5 centers listed in the author’s information, 949 patients were identified and assessed for eligibility. Of these, 875 were deemed ineligible, leaving 74 (7.79%) patients eligible to undergo treatment: 37 to undergo D1 resection, and 37 to undergo D2 resection. The method used for patient selection is shown in Figure 1. The patients underwent D2 lymphadenectomy as part of standard radical gastrectomy. To best serve our research objective, the number of lymph nodes removed assisted in classifying lymphadenectomy. A total number of lymph nodes greater than 15 in the pathology report was defined as adequate D2, while a total less than or equal to 14 was defined as D1.
A D1 dissection involves the affected distal section of the stomach or the stomach in its entirety (subtotal or total resection) being removed, including the greater and lesser omenta (7-9). D2 dissection, meanwhile, sees the removal of the omental bursa as well as the front leaf of the transverse mesocolon, and the mentioned arteries are totally cleared. Resection of the spleen and the tail of the pancreas was considered to be required for the satisfactory removal of D2 lymph-node stations 10 and 11 in proximal tumors.
In contrast, in a D1 dissection, the spleen and tail of the pancreas resections were carried out only in instances where removal was deemed necessary due to tumor invasion. Following the last pathological examination, the surgery was classified as R0 if the tumor was shown by microscopic evidence to have been entirely removed, if distant lymph nodes were not found to be involved, and if no malignant cells were discovered in the abdominal-washing fluid. No distal pancreatectomy and/or splenectomy was performed during D1 or D2 resection unless there was direct tumor invasion to the two organs at the time of presentation.
The percentage of positive node involvement (D+ %) was calculated as positive lymph node among total dissected lymph nodes. The positive node was defined as invasion in the dissected specimen, and pathologically confirmed lymph node involvement. Nodes with unknown status were excluded in the D+ % evaluation.
Patients who did not meet the criteria were excluded in this study. The criteria were individuals under the age of 75 years of age with histologically proven gastric adenocarcinoma, which was surgically removed with the D1 or D2 techniques. Further eligibility criteria, stemming from the intraoperative findings, were the detection of a stage T2b, T3, or T4 tumor, the absence of gross metastases in the abdominal cavity, and negative cytologic findings in the peritoneal-lavage fluid. All positive lymph nodes were diagnosed by dissection, not by examination of frozen sections. We ensured quality control by relating the number and location of lymph nodes identified through pathological examination. The detection of lymph nodes in stations besides those specified, or an inability to detect lymph nodes in stations that should have been dissected, led to the case being classified as unknown LN. The amounts of lymph nodes varied significantly at each station, and those stations defined may not have contained any lymph nodes (10,11). The study protocol was approved by the Ethics Committee of Sichuan Provincial People’s Hospital. Informed consent was obtained from all patients before surgery was performed. For the purpose of the study, written consent was waived owing to the study’s retrospective nature. Patients who underwent minimal invasive or open laparotomy were included in the study.
The clinical pathology, operative, and survival data were compared between the D1 and D2 groups. Patients were considered to have undergone additional organ resection when there was a record of the procedure in the patient’s operative note or the pathological report. All disease stages were assigned using the 2017 American Joint Committee on Cancer TNM staging guidelines.
Chi-square analysis was applied to make comparisons based on the clinical pathology data. Parameters that influenced survival and recurrence were compared through the use of the Kaplan-Meier method with the log-rank comparison. A significant difference was indicated when P value =0.05. The results of significance between variables via univariate and multivariate analysis were not presented due to the small cohort size.
Results
A balance was maintained between the two groups in relation to age, sex, tumor size, the extent of resection, and pathology (Table 1). The median number of positive LNs removed from the D2 arm was significantly larger than in the D1 arm (11.19% vs. 9.03%, respectively; P<0.007).
Table 1
| Characteristic | D1 | D2 | t/χ2 | P value† |
|---|---|---|---|---|
| Age (year) | −0.246 | 0.806 | ||
| Mean | 55.89 | 56.08 | ||
| SD | 3.41 | 3.19 | ||
| Median | 56 | 57 | ||
| Range | 51–67 | 51–63 | ||
| Sex, n | 0.000 | 1.000 | ||
| F | 18 | 18 | ||
| M | 19 | 19 | ||
| Tumor location, n | 0.897 | 0.639 | ||
| Upper | 8 | 7 | ||
| Mid | 15 | 19 | ||
| Low | 14 | 11 | ||
| Tumor size¶ (cm) | 0.689 | 0.493 | ||
| Mean | 6.51 | 6.06 | ||
| SD | 2.94 | 2.61 | ||
| Median | 6 | 6 | ||
| Range | 1–13 | 1.7–14 | ||
| Clinical stage, n | – | 0.736 | ||
| T1–2 | 6 | 4 | ||
| T3–4 | 31 | 33 | ||
| Pathological stage, n | – | 1.000 | ||
| T1–2 | 5 | 5 | ||
| T3–4 | 32 | 32 | ||
| No. of positive lymph nodes | 2.821 | 0.007 | ||
| Mean | 9.03 | 11.19 | ||
| SD | 1.99 | 4.22 | ||
| Median | 9 | 10 | ||
| Range | 7–14 | 7–27 | ||
| R resection*, n | 0.214 | 0.643 | ||
| R0 | 34 | 35 | ||
| R1 | 3 | 2 | ||
| Type of resection, n | 0.840 | 0.359 | ||
| Total | 8 | 5 | ||
| Subtotal | 29 | 32 | ||
¶, the T stage was determined according to the AJCC 2016 edition; †, P values were calculated using Fisher’s exact test except for comparisons of age, tumor size, and number of positive lymph nodes; *, R0, no microscopic residual disease, R1 with microscopic residual disease.
No significant difference in postoperative morbidity was noted between the D1 (10.81%, n=4) and D2 treatment arms (18.9%, N=7) (P=0.327) (Table 2). Death within 90 days of surgery was considered as postoperative mortality, and a total of 3 patients died during this period: 1 (2.7%) from the D2 group and 2 (5.4%) from the D1 group (Table 2).
Table 2
| Postoperative morbidity | Outcome* | P value | |
|---|---|---|---|
| D1 | D2 | ||
| Total morbidity, n | 4 | 7 | 0.327 |
| Total death, n (%) | 1 (2.7) | 2 (5.4) | 1.000 |
*, calculated using Fisher’s exact test.
The most common site of recurrence was the peritoneum in both arms. The first site of recurrence was not significantly different between D1 (n=16, 43.2%) and D2 (n=19, 51.3%). Recurrences at the anastomotic site were less common in both groups (D1: n=5, 13.5% vs. D2: n=4, 10.8%). Overall, the rate of recurrence was higher in D1 (n=35, 94.5%) than in D2 (n=31, 83.7%) (Table 3).
Table 3
| Site | D1, n (%) | D2, n (%) | P value |
|---|---|---|---|
| Anastomosis site | 5 (13.5) | 4 (10.8) | 1.000 |
| Peritoneal/lymph node | 16 (43.2) | 19 (51.3) | 0.642 |
| DM (liver or lung) | 7 (18.9) | 5 (13.5) | 0.528 |
| Other | 7 (18.9) | 3 (8.1) | 0.174 |
| Total | 35 (94.5) | 31 (83.7) | 0.261 |
DM, distant metastasis.
Time to recurrence
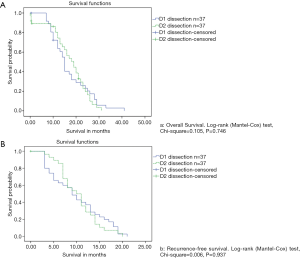
There was a median follow-up period of 24 months (ranging from 7 to 40 months) for all patients who underwent R0 resection. A total of 66 (89.1%) patients in the entire population had recurrences, of which 44 (59.4%) were local, and 12 (16.2%) were distant. The median disease-free survival (DFS) was 9.72 and 7.81 months for the D2 and D1 groups, respectively (P=0.746). Meanwhile, the median OS was 16.39 and 15.85 months for the D2 and D1 groups, respectively (P=0.937). The incidence of recurrence between the two dissection groups was not significantly different (D1: 35/37, 94.5% vs. D2: 31/37, 83.7%; P=0.261). The median time to recurrence was 10 and 9 months in D2 and D1 groups, respectively. Among patients who underwent adjuvant therapy, the time to recurrence was significantly different between the two groups. Adjuvant treatment prolonged the recurrence from 6 months to 15 months and from 5 months to 14 months in the D2 and D1 groups, respectively.
Survival
There was a median survival period of 15 months for the group as a whole. After excluding the patients lost to follow-up, the length of survival in the D1 group (median: 17 months) was not significantly better than in the D2 group (median: 15 months) (Figure 2). The median survival time for patients who underwent splenectomy was 15 months, and this rose to 16 months for patients who underwent pancreatosplenectomy. Analysis based on the disease stage (stages II to IV) revealed that survival was not significantly decreased by the removal of additional nodes in both D2 and D1 (Figure 2). Survival was not affected by the specific type of organ to be resected within each stage.
Discussion
Using multi-center data, we compared patients with N3 gastric cancer who underwent two types of surgical procedure, D1, and D2 curative resection, and found no significant differences in outcomes following surgery in these patients.
Primary surgery followed by adjuvant treatment remains the standard treatment for N3 gastric cancer, and neoadjuvant chemotherapy or chemoradiation in our study period was not the standard form of care. To avoid bias, patients who had received neoadjuvant treatment were excluded from the study. Overall, approximately 8% of patients in our cohort had N3, which is consistent with previous reports (12,13).
Our cohorts were all recruited from tertiary hospitals where more than 100 gastrectomy surgeries are performed annually. All of the surgeons were well trained, and each had more than 10 years’ experience in related procedures. The volume-related quality issue raised by some authors (14) did not apply in this study.
The rate of surgical morbidity or mortality was not different between the D1 and D2 groups. Two large phases III trials—DGCT and MRC—reported high mortality rates after D2 dissection (15-17).
Our data did not show that extensive resection may have an adverse effect on prognosis. Zhang and Masahiro attributed this phenomenon to local tumor spillage from the many divided lymphatic vessels (17,18). The mortality rate in the present study was consistent with that reported by Degiuli and Talaiezadeh (19,20). The inconsistency in the rate of mortality in the two groups is likely due to differences in cohort size and types of analysis carried out. Future extended studies to confirm our findings are called for.
Studies have reported potential survival benefits from D2 dissection (3,6,21,22), which is inconsistent with our findings. However, those studies included patients from different populations and enrolled only those in the early stage of the disease. In their cohorts, patients with N3 accounted for less than 10%, and it is unclear whether the results of the subgroup analyses of the N3 patients could yield the same conclusion as those of the entire cohorts. Based on our results, we hypothesize that the extension of D2 lymph node dissection might hold benefits only as a treatment for early-stage diseases such as N1 or N2, but not for N3 or later-stage disease. This could be because N3 disease is already systemic; as such, neither regional control nor survival can be influenced by intensive local lymph node dissection.
In China, the OS of gastric cancer patients has remained low over recent decades, and the 5-year cancer-specific mortality rate for stage III gastric cancer is higher than that in the United States, with approximately 72% vs. 56%, respectively (13). In contrast, in the same period, the Japanese Research Society for Gastric Cancer reported improved survival figures, with an OS of approximately 50% after resection (23). During this time, an increasing number of early-stage gastric cancer cases have been diagnosed via population-screening programs (24). Collectively, these improvements explain the improved Japanese survival data, as patients were diagnosed earlier. However, another study has suggested that the improved survival rate seen in Japan can be attributed to the adoption of radical surgery with lymphadenectomy (6). Such treatment modality was implemented in Japan based on the theory that effective locoregional control of cancer reduces recurrence in the gastric bed. If this theory is true, then extensive lymph node dissection would provide better local control for N3 disease.
However, evidence of the clinical benefit of extensive D2 lymph node dissection for locally advanced gastric cancer (LAGC) and N3 disease is lacking; thus, its role in the treatment of such patients remains controversial. Previous randomized control trials enrolled patients that have either N1 or N2 disease, and only a few have included those with N3 disease. To our knowledge, our study is the first to have focused on patients with N3 disease and compared the clinical benefit of D1 lymphadenectomy with D2 lymphadenectomy for gastric cancer. Our data show that patients with N3 disease may not benefit from D2 extensive node dissection.
Future efforts to improve the survival of patients with LAGC must concentrate on a multidisciplinary approach. The MAGIC study has demonstrated the survival benefits of perioperative chemotherapy for LAGC (25), and the ongoing TOPGEAR and President studies are expected to provide more data on the therapeutic value of neoadjuvant chemoradiation for treating gastric cancer (26).
This study had some limitations, including its retrospective nature and inconsistencies in data from the post-treatment review. Moreover, not all stations for positive nodes were matched, and a previous report showed that metastases in the station are predictive of OS (27).
In summary, our study demonstrated that patients with gastric cancer who have a high number of positive lymph nodes have poor outcomes. Moreover, extensive node dissection did not improve the outcomes of such patients. Although Sasako et al. reported that extensive node dissection led to worse prognosis in node-positive patients (6), their study included patients with early-stage disease. Our findings should be interpreted with caution because this is a retrospective review and was thus subject to biases and errors and because there was no accurate manner in which to assess lymph-node metastases prior to surgery and intraoperative frozen-section diagnosis of all dissected lymph nodes was not feasible.
Given that our results show that D2 extended lymphadenectomy does not improve outcomes in patients with N3 gastric cancer, further research is needed to determine an alternative approach to improve the outcomes of these patients.
Acknowledgments
Funding: This study was funded by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.03.42). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was reviewed and approved by the Ethics Committee of Sichuan Provincial People’s Hospital (No: 2017-126-7). Patients were not required to give informed consent to the study because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kelley JR, Duggan JM. Gastric cancer epidemiology and risk factors. J Clin Epidemiol 2003;56:1-9. [Crossref] [PubMed]
- Uslu A, Zengel B, Ilhan E, et al. Survival outcomes after D1 and D2 lmphadenectomy with R0 resection for stage II-III gastric cancer: Longitudinal follow-up in a single center. Turk J Surg 2018;34:125. [Crossref] [PubMed]
- Mogal H, Fields R, Maithel SK, et al. In Patients with Localized and Resectable Gastric Cancer, What is the Optimal Extent of Lymph Node Dissection-D1 Versus D2 Versus D3? Ann Surg Oncol 2019;26:2912-32. [Crossref] [PubMed]
- Kong SH, Lee HJ, Ahn HS, et al. Stage Migration Effect on Survival in Gastric Cancer Surgery With Extended Lymphadenectomy: The Reappraisal of Positive Lymph Node Ratio as a Proper N-Staging. Ann Surg 2012;255:50-8. [Crossref] [PubMed]
- Laterza E, Giacopuzzi S, Minicozzi A, et al. Significance of super-extended (D3) lymphadenectomy in gastric cancer surgery Ann Ital Chir 2009;80:101-6. [PubMed]
- Sasako M, Sano T, Yamamoto S, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med 2008;359:453-62. [Crossref] [PubMed]
- Karpeh MS, Leon L, Klimstra D, et al. Lymph node staging in gastric cancer: is location more important than number? An analysis of 1,038 patients. Ann Surg 2000;232:362-71. [Crossref] [PubMed]
- American Joint Committee on Cancer. AJCC Cancer Staging Manual, 2017.
- Ramos MFKP, Pereira MA, Dias AR, et al. Surgical outcomes of gastrectomy with D1 lymph node dissection performed for patients with unfavorable clinical conditions. Eur J Surg Oncol 2019;45:460-5. [Crossref] [PubMed]
- Bunt AMG, Hermans J, Boon MC, et al. Evaluation of the extent of lymphadenectomy in a randomized trial of Western- versus Japanese-type surgery in gastric cancer. J Clin Oncol 1994;12:417-22. [Crossref] [PubMed]
- Zhang W, Zhangyuan G, Wang J, et al. Effect of lymph nodes count in node-positive gastric cancer. J Cancer 2019;10:5646-53. [Crossref] [PubMed]
- Martin RC 2nd, Jaques DP, Brennan MF, et al. Extended Local Resection for Advanced Gastric Cancer Increased Survival Versus Increased Morbidity. Ann Surg 2002;236:159-65. [Crossref] [PubMed]
- Strong VE, Wu AW, Selby LV, et al. Differences in gastric cancer survival between the U.S. and China. J Surg Oncol 2015;112:31-7. [Crossref] [PubMed]
- Krijnen P, den Dulk M, Meershoek-Klein Kranenbarg E, et al. Improved survival after resectable non-cardia gastric cancer in the Netherlands: the importance of surgical training and quality control. Eur J Surg Oncol 2009;35:715-20. [Crossref] [PubMed]
- Hartgrink HH, Jansen EP, van Grieken NC, et al. Gastric cancer - seminar. Lancet 2009;374:477-90. [Crossref] [PubMed]
- Zhu Z, Wu P, Du N, et al. Surgical choice of proximal gastric cancer in China: a retrospective study of a 30-year experience from a single center in China. Expert Rev Gastroenterol Hepatol 2019;13:1123-8. [Crossref] [PubMed]
- Zhang W, Zhangyuan G, Wang J, et al. Effect of lymph nodes count in node-positive gastric cancer. J Cancer 2019;10:5646-53. [Crossref] [PubMed]
- Watanabe M, Kinoshita T, Morita S, et al. Clinical impact of splenic hilar dissection with splenectomy for gastric stump cancer. Eur J Surg Oncol 2019;45:1505-10. [Crossref] [PubMed]
- Degiuli M, Sasako M, Ponti A, et al. Randomized clinical trial comparing survival after D1 or D2 gastrectomy for gastric cancer. Br J Surg 2014;101:23-31. [Crossref] [PubMed]
- Talaiezadeh AH, Asgari M, Zargar M, et al. Mortality and Morbidity and Disease Free Survival after D1 and D2 Gastrectomy for Stomach Adenocarcinomas. Asian Pac J Cancer Prev 2015;16:5253-6. [Crossref] [PubMed]
- Fujita J, Kurokawa Y, Sugimoto T, et al. Survival benefit of bursectomy in patients with resectable gastric cancer: interim analysis results of a randomized controlled trial. Gastric Cancer 2012;15:42-8. [Crossref] [PubMed]
- Jiang L, Yang KH, Guan QL, et al. Survival and recurrence free benefits with different lymphadenectomy for resectable gastric cancer: A meta-analysis. J Surg Oncol 2013;107:807-14. [Crossref] [PubMed]
- Nio Y, Tsubono M, Kawabata K, et al. Comparison of Survival Curves of Gastric Cancer Patients After Surgery According to the UICC Stage Classification and the General Rules for Gastric Cancer Study by the Japanese Research Society for Gastric Cancer. Ann Surg 1993;218:47-53. [Crossref] [PubMed]
- Kawai K, Misaki F. Early Gastric Cancer. Cancer Invest 1982;77-8.
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Leong T, Smithers BM, Michael M, et al. TOPGEAR: a randomised phase III trial of perioperative ECF chemotherapy versus preoperative chemoradiation plus perioperative ECF chemotherapy for resectable gastric cancer (an international, intergroup trial of the AGITG/TROG/EORTC/NCIC CTG). BMC Cancer 2015;15:532. [Crossref] [PubMed]
- Li C, Kim S, Lai JF, et al. Risk Factors for Lymph Node Metastasis in Undifferentiated Early Gastric Cancer. Ann Surg Oncol 2008;15:764-9. [Crossref] [PubMed]