
Prognosis of stage III cervical cancer: a two-way outcome study
Introduction
With an estimated 570,000 new cases and 311,000 deaths in 2018, cervical cancer is the second most common female malignancy worldwide; 1 in 75 women develop cervical cancer during a lifetime (1). In China, cervical cancer is the second leading cause of cancer death among women, and there were over 102,000 new cases and 30,000 deaths in 2014 (2), making it a major public health problem. Approximately two-thirds of patients in developing countries have locally advanced stage disease at diagnosis, and the prognosis of these patients is poor, with a high risk of metastasis or relapse (3,4). Although the 5-year survival rate of stage III cervical cancer has increased by 30–35% in recent years (5), its prognosis is still unsatisfactory.
Although randomized controlled trials (RCTs) are regarded as the best study design for producing evidence of an intervention, ethical limitations and the generalization of their findings restrict the application of this design, and it is difficult to interpret the complex and diverse clinical scenarios associated with their findings (6,7). In contrast, with the increase in the digitization, availability, and accessibility of clinical information, outcome studies based on electronic medical records and other resources have been widely performed (8,9). Such studies on the prognosis of cervical cancer have mainly focused on treatment effectiveness or death by analyzing the influence of various clinicopathological factors (10,11). In this study, we used the two-way outcome study method to comprehensively analyze information from patients with stage III cervical cancer to determine the effect factors that influence overall survival (OS), progression-free survival (PFS), and quality of life, thus providing evidence for the improvement of the prognosis of these patients.
Methods
Patients
Patients with cervical cancer who were treated at Harbin Medical University Cancer Hospital from 1 January 2013 to 31 December 2015 were enrolled. The inclusion criteria were: International Federation of Gynecology and Obstetrics (FIGO) stage III cervical cancer; no other malignant tumors at diagnosis; and initial treatment at this hospital. The study was approved by the ethics committee at Harbin Medical University in Harbin, China.
Data collection
Case information collected during the first treatment were extracted from the Health Records Management System (Microsoft SQL Server 2012) using the Visual C# language and were associated by unique variable identification (ID). The required data were converted from text format and were entered into a database established using Epi Info 3.5.1; the required data included data of demographic characteristics, clinical stage, histological morphology, auxiliary examination, and treatment. Weight change and mental status were based on the patients’ complaint and doctor’s assessment at the time of admission. Tumor diameter and lymph node status were assessed by magnetic resonance imaging and computed tomography at baseline.
Treatment
External beam radiotherapy (EBRT) was performed with a dose of 1.8 Gy per fraction five times per week for a total dose of 45–51 Gy; the dose was increased to 63.5 Gy in areas that were lymph-node-positive. Intra-cavity brachytherapy (ICBT) was delivered to point A (a reference location 2 cm lateral and 2 cm superior to the cervical os) once weekly during EBRT, and the total dose was 30 Gy. Chemotherapy was administered according to the following regimens: weekly platinum drugs or plus paclitaxel every 3 weeks. If the functions of the liver, kidney, and heart were too poor to tolerate chemotherapy or if the patients had other considerations, radiotherapy alone was performed.
Follow-up
From treatment completion until June 2017, the patient follow-up included hospital review and a questionnaire-based telephone survey. Hospital review was scheduled every 3 months for the first year and every 6 months from the second to the fifth years. Routine and gynecologic examinations were performed at each appointment to track disease progression. Suspected cases of recurrence were confirmed by biopsy or MRI. The questionnaire-based telephone survey was conducted by pre-trained investigators each year. The administered questionnaire was based on the Functional Assessment of Cancer Therapy-Cervix and other related scales, and it comprised five dimensions including the patient’s survival status, disease progression, physical status, social/family status, and mental status. The summary score ranged from 0 to 100, where a higher score indicated a better quality of life. Family care, work load, and number of reviews 1 year after treatment were considered as independent variables.
Outcomes measures
The primary outcome measure was OS. Secondary outcomes included PFS and patient-reported quality of life. OS was defined as the time from diagnosis to cervical cancer-related death, and PFS was defined as the time from diagnosis to recurrence (including local relapse and distant metastasis) or all-cause death. Local relapse meant that the recurrent tumor was located in the pelvic cavity, and distant metastasis was defined as a recurrent tumor that was located in tissues or organs outside the pelvic cavity.
Statistical analysis
Data sorting, cleaning, and all statistical analyses were performed using SPSS 17.0. Uni- and multi-variate analyses of OS and PFS were performed using the Kaplan-Meier method and Cox proportional hazards regression model, respectively. Analysis of variance, linear correlation, and multiple linear regression was used for uni- and multi-variate analyses of quality of life. P values <0.05 were considered statistically significant.
Results
Patient characteristics
The patients’ characteristics are summarized in Table 1. A total of 460 subjects were enrolled in this study, and 32 patients (7.0%) were younger than 40 years. Among them, the major histological subtype was squamous cell carcinoma (SCC; 93.3%); 60.4% of the patients presented with bulky tumors (≥4 cm), and 41.7% were lymph-node-positive. In total, 341 patients (74.1%) underwent concurrent chemoradiotherapy (CCRT), 92 patients (20%) received treatments other than CCRT, and 27 patients (5.9%) did not complete standardized treatment.
Table 1
| Variable | Subgroup | N | % |
|---|---|---|---|
| Age at diagnosis (years) | ≤40 | 32 | 7.0 |
| 41–50 | 135 | 29.3 | |
| 51–60 | 174 | 37.8 | |
| 61–70 | 92 | 20.0 | |
| >70 | 27 | 5.9 | |
| Medical insurance | Urban | 250 | 54.3 |
| Rural | 153 | 33.3 | |
| Private expense | 35 | 7.6 | |
| Others | 22 | 4.8 | |
| Pregnancy (times) | 0 | 23 | 5.0 |
| 1–2 | 196 | 42.6 | |
| 3–4 | 172 | 37.4 | |
| 5 | 69 | 15.0 | |
| Primiparous age (years) | <20 | 61 | 13.3 |
| 20–24 | 214 | 46.5 | |
| 25–29 | 139 | 30.2 | |
| ≥30 | 46 | 10.0 | |
| Menopausal status | Yes | 276 | 60.0 |
| No | 184 | 40.0 | |
| Abortion (times) | 0 | 188 | 40.9 |
| 1–2 | 230 | 50.0 | |
| >2 | 42 | 9.1 | |
| Hypertension | Yes | 60 | 13.0 |
| No | 400 | 87.0 | |
| Heart disease | Yes | 38 | 8.3 |
| No | 422 | 91.7 | |
| Family history | Yes | 40 | 8.7 |
| No | 420 | 91.3 | |
| Histology | SCC | 429 | 93.3 |
| Adenocarcinoma | 19 | 4.1 | |
| Others | 12 | 2.6 | |
| Tumor diameter (cm) | <4 | 182 | 39.6 |
| ≥4 | 278 | 60.4 | |
| LNM | Positive | 192 | 41.7 |
| Negative | 268 | 58.3 | |
| CCRT | Yes | 341 | 74.1 |
| No | 92 | 20.0 | |
| Incomplete | 27 | 5.9 |
LNM, lymph nodal metastasis; SCC, squamous cell carcinomas; CCRT, concurrent chemotherapy radiotherapy.
Follow-up
The median follow-up time was 28 months (range, 1–51 months). At the end of the follow-up, survival status and disease progression were assessed in the 460 patients: 119 patients died of cervical cancer-related causes (75 of these patients showed recurrence); of the 341 survivors, the number of patients with recurrence was 54, and 65 patients were lost to follow-up during the telephone interview; thus, the quality of life was unknown.
Survival analysis
The 3-year OS and PFS rates were 69.0% and 55.0%, respectively (Figure 1). As assessed by the Kaplan-Meier method, age at diagnosis (P=0.011), primiparous age (P=0.001), weight change (P<0.001), mental status (P=0.004) at admission, tumor diameter (P=0.030), lymph node metastasis (LNM; P<0.001), and CCRT use (P<0.001) were identified as significant factors for OS (Table 2 and Figure 2). Similar correlation results were obtained for PFS, but PFS was also significantly affected by histology (P=0.032) (Table 2 and Figure 3). Among the above factors, LNM, weight change, and CCRT use were included in the Cox proportional hazards regression model in Table 3. Only weight change was associated with OS.
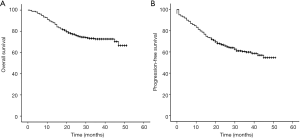
Table 2
| Variables | Subgroups | Number | OS | PFS | |||||
|---|---|---|---|---|---|---|---|---|---|
| Case | χ2 | P value | Case | χ2 | P value | ||||
| Age at diagnosis (years) | ≤40 | 32 | 14 | 6.538 | 0.011 | 19 | 8.887 | 0.003 | |
| >40 | 428 | 105 | 154 | ||||||
| Primiparous age (years) | <30 | 414 | 98 | 11.609 | 0.001 | 148 | 7.629 | 0.006 | |
| ≥30 | 46 | 21 | 25 | ||||||
| Histology | SCC | 429 | 107 | 2.893 | 0.089 | 156 | 4.577 | 0.032 | |
| Others | 31 | 12 | 17 | ||||||
| Tumor diameter (cm) | <4 | 182 | 37 | 4.736 | 0.030 | 56 | 6.164 | 0.013 | |
| ≥4 | 278 | 80 | 115 | ||||||
| LNM | Positive | 192 | 69 | 17.524 | <0.001 | 98 | 27.077 | <0.001 | |
| Negative | 268 | 50 | 75 | ||||||
| Weight | Not change | 34 | 19 | 29.287 | <0.001 | 21 | 14.439 | <0.001 | |
| Lose weight | 426 | 100 | 152 | ||||||
| Mental status | Good | 161 | 30 | 8.103 | 0.004 | 47 | 8.471 | 0.004 | |
| General | 273 | 80 | 114 | ||||||
| Poor | 26 | 9 | 12 | ||||||
| CCRT | Yes | 341 | 69 | 52.519 | <0.001 | 107 | 43.478 | <0.001 | |
| No | 92 | 33 | 47 | ||||||
| Incomplete | 27 | 17 | 19 | ||||||
LNM, lymph nodal metastasis; CCRT, concurrent chemotherapy radiotherapy; OS, overall survival; PFS, progression−free survival.
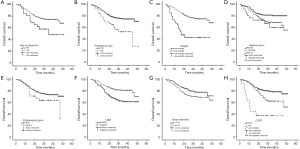

Table 3
| Variables | OS | PFS | |||||
|---|---|---|---|---|---|---|---|
| P value | HR | 95% CI | P value | HR | 95% CI | ||
| LNM | <0.001 | 0.354 | 0.234–0.534 | <0.001 | 0.402 | 0.286–0.564 | |
| Weight | 0.003 | 0.422 | 0.238–0.750 | 0.172 | 0.690 | 0.405–1.175 | |
| CCRT | <0.001 | <0.001 | |||||
| No | 0.173 | 1.389 | 0.866–2.228 | 0.083 | 1.415 | 0.955–2.095 | |
| Incomplete | <0.001 | 8.348 | 4.534–15.370 | <0.001 | 5.309 | 2.996–9.408 | |
LNM, lymph nodal metastasis; CCRT, concurrent chemotherapy radiotherapy; OS, overall survival; PFS, progression−free survival; HR, hazard ratio; CI, confidence interval.
Quality of life
The scores of overall quality of life, disease status, physical status, social/family status, and mental status for the 276 survivors were 85.4±10.0, 31.1±3.0, 32.8±4.6, 12.0±2.3, and 8.5±2.8, respectively; all modules were significantly related. The factors related to overall quality of life were age at diagnosis, disease progression, family care, work load, and number of reviews 1 year after treatment. Among these factors, family care and disease progression exerted an influence on all dimensions (Table 4). Next, multivariate linear regression analysis was performed using overall quality of life as a dependent variable, and the factors in Table 5 were included as independent variables. Disease progression, age at diagnosis, primiparous age, and work load 1 year after treatment were independent determinants of overall quality of life and explained 28.9% of the variation (Table 5).
Table 4
| Variables | Overall conditions | Disease condition | Physiological state | Social/family state | Mental state | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r/Fa | P value | r/F | P value | r/F | P value | r/F | P value | r/F | P value | |||||
| Continuous (r) | ||||||||||||||
| Age at diagnosis | −0.136 | 0.024 | −0.060 | 0.323 | −0.175 | 0.004 | −0.246 | <0.001 | 0.059 | 0.327 | ||||
| Menarche age | −0.098 | 0.104 | −0.012 | 0.845 | −0.155 | 0.010 | −0.104 | 0.083 | −0.001 | 0.981 | ||||
| Marry age | −0.104 | 0.084 | −0.032 | 0.592 | −0.080 | 0.186 | −0.069 | 0.251 | −0.152 | 0.011 | ||||
| Primiparous age | −0.099 | 0.109 | −0.006 | 0.918 | −0.069 | 0.260 | −0.101 | 0.100 | −0.150 | 0.014 | ||||
| Age at last delivery | −0.112 | 0.068 | −0.028 | 0.652 | −0.150 | 0.014 | −0.129 | 0.035 | −0.042 | 0.499 | ||||
| Categorical (F) | ||||||||||||||
| LNM | 3.142 | 0.077 | 6.081 | 0.014 | 2.017 | 0.157 | 0.283 | 0.595 | 0.796 | 0.373 | ||||
| Medical insurance | 2.149 | 0.094 | 1.661 | 0.176 | 2.183 | 0.090 | 0.097 | 0.961 | 3.183 | 0.024 | ||||
| Review times | 3.453 | 0.017 | 4.991 | 0.002 | 2.507 | 0.059 | 3.223 | 0.023 | 1.022 | 0.383 | ||||
| Distant metastasis | 30.644 | <0.001 | 161.107 | <0.001 | 8.65 | 0.004 | 6.826 | 0.009 | 2.326 | 0.128 | ||||
| Relapse | 53.858 | <0.001 | 219.143 | <0.001 | 14.511 | <0.001 | 14.167 | <0.001 | 8.984 | 0.003 | ||||
| Family care | 4.26 | 0.018 | 6.025 | 0.004 | 3.361 | 0.041 | 6.546 | 0.003 | 6.366 | 0.003 | ||||
| Work volume | 6.996 | <0.001 | 3.877 | 0.010 | 6.173 | <0.001 | 7.742 | <0.001 | 1.86 | 0.137 | ||||
a, r/F: r = correlation coefficient; F = F value of analysis of variance. LNM, lymph nodal metastasis.
Table 5
| Variables | Regression coefficient | Standard regression coefficient | T value | P value |
|---|---|---|---|---|
| Constant term | 109.883 | 17.388 | <0.001 | |
| Relapse | −13.190 | −0.357 | 6.331 | <0.001 |
| Distant metastasis | −7.622 | −0.219 | 3.902 | <0.001 |
| Age at diagnosis | −0.198 | −0.168 | −3.010 | 0.003 |
| Primiparous age | −0.477 | −0.142 | −2.499 | 0.013 |
| Work volume | ||||
| >50% | −1.384 | −0.061 | −1.072 | 0.285 |
| ≤50% | −3.336 | −0.124 | −2.205 | 0.028 |
| None | −7.016 | −0.173 | −3.078 | 0.002 |
Model parameters: F=8.024, P<0.001, R2=0.289.
Discussion
Recently, the OS of stage III cervical cancer has increased by 30–35% (5). A previous study reported that the 3-year OS and PFS rates were 61.3% and 54.8% for stage III disease (12). In the present study, the 3-year OS and PFS rates were 69.0% and 55.0%, respectively. The observation that the OS rate was higher than that in previous studies may be attributable to the fact that some patients were followed-up for less than 3 years.
LNM, non-SCC, and larger tumors were previously reported as poor prognostic factors for cervical cancer (13-15). This study had similar results, but only histology had a significant effect on PFS. This result may be explained by the small number of patients with non-SCC. CCRT is established as the standard treatment for patients with locally advanced cervical cancer as it improves OS and PFS rates (16-18). However, we found that although CCRT could improve OS and PFS rates, there was no significant difference in outcomes between patients with and without CCRT (Table 3). The reason may be that there was an intersection in the survival curve between two groups (Figures 2H,3H). The OS and PFS rates of the two groups were similar within 12 months, with the CCRT group having slightly lower values, possibly because the incidence of adverse reactions increased during CCRT. However, similar to that in the abovementioned previous studies, the OS and PFS after 1 year were significantly higher in the CCRT group than in the no-CCRT group.
Body mass index (BMI) itself was not identified as a prognostic factor for OS and PFS in this study. This was inconsistent with the results reported by a previous study (19). However, we found that weight loss was a protective factor. At admission, patients who complained of weight loss had higher BMI than those without weight loss; thus, when they lost weight, the BMI tended to be normal, which might prevent the occurrence of adverse outcomes to some extent. The mental state of the patients at admission was also a prognostic factor. The worse the mental state of the patients at admission, the worse was their prognosis. One reason for this phenomenon might be the fact that poor mental state might exacerbate the patient’s physical burden, thus increasing the risk of adverse outcomes.
The median age of the enrolled patients was 54 years. In total, 67.1% patients were aged between 40 and 60 years, and this age group was consistent with the age of high cervical cancer incidence. Whether age at diagnosis is a prognostic risk factor of cervical cancer is controversial, and most studies have indicated that the prognosis of young patients with locally advanced disease is worse than that of older patients (20,21). In this study, patients younger than 40 years were more susceptible to disease progression, and their median OS and PFS were 35 and 23 months, respectively, which were lower than those of older patients.
In this study, primiparous age above 30 years was a prognostic risk factor in patients with stage III cervical cancer. Previous reviews have strongly suggested that marital history, such as younger age at marriage and childbirth, multiple pregnancies, and multiple births, were risk factors for cervical cancer incidence (22,23). However, the impact of these factors on prognosis has been seldom reported. We found that patients with primiparous age above 30 years were characterized by older age at marriage and the last childbirth, fewer pregnancies and births, and younger age at diagnosis compared with other patients. The increased risk of incidence and poor prognosis may thus be explained by increased burden of the uterus and other body functions with increased primiparous age and the increased levels of sex hormones and decreased immunity during pregnancy (24,25). We speculate that these factors may be among the explanations for the recurrence of cervical cancer. Future in-depth studies are needed to clarify this issue.
The focus of the cancer control strategy proposed by World Health Organization in 2005 is not only the reduction of cancer cases but also the improvement of the quality of life of patients and their families (26). To some extent, quality of life is more important than OS and PFS, especially for patients with advanced cancer. In this study, the quality of life score was higher than those in previous studies (27,28). This is likely because patients might intentionally avoid adverse options when self-reporting. Age at diagnosis, number of reviews, family care, and work load were associated with quality of life in univariate analysis (Table 4). The risk of chronic disease in patients increases with age, which might lead to a reduction in their quality of life. Frequent review, according to medical orders, is useful in detecting the patients’ conditions in a timely manner, thus effectively preventing the occurrence of adverse outcomes. Good care from family members and returning to work within 1 year after treatment can improve a patient’s sense of social identity. Multivariate analysis (Table 5) showed that the quality of life decreased with increased primiparous age, which may be explained by reasons similar to those discussed for the survival analysis. In this study, there was no correlation between clinicopathologic factors and quality of life, possibly because of the low survival rate of patients with high-risk factors. Considering the patients’ privacy and resistance, we did not analyze the association of quality of life with economic conditions and family burdens, considered to be influencing factors in previous studies (29,30). However, the relationship between medical insurance and the quality of life was analyzed, showing that this factor was only related to the mental state of patients.
Importance of the study
This was an outcome study aiming to achieve bidirectional translation between practice and theory. Regarding the translation from practice to theory, most data sources of outcome studies are electronic medical records and insurance databases, which were established at an earlier date in developed countries. In China, the digitization of electronic medical records began comparatively late and developed slowly. Moreover, the database systems vary, not only at individual hospitals but also between different hospitals. These factors have resulted in low availability of, accessibility to, and utilization of real-world data. However, in this study, we overcame these difficulties to merge medical record files in different formats. All databases in the Health Records Management System were integrated and converted to the required data format, thus ensuring comprehensiveness and consistency, while telephone follow-ups were conducted to ensure the accuracy of data.
As for translating from theory to practice, we found that primiparous age above 30 years, age at diagnosis below 40 years, LNM, non-SCC, and larger tumors were risk factors of OS and PFS, while the protective factors were weight loss, good mental status, and standardized treatment. The quality of life was better in patients with the following characteristics: younger age at diagnosis, active review according to medical orders, and earlier return to society. Patients with these high-risk factors should undergo enhanced intervention and be actively followed-up to prolong survival and improve their quality of life.
Strengths and limitations
This study was an outcome study exploring not only OS but also the PFS and quality of life of patients with stage III cervical cancer. Despite being observational in nature, the two-way outcome study design, including a retrospective investigation and prospective follow-up, overcame some of the limitations of RCTs. The limitations of this study are the general limitations of an observational study and its single-center design, although the sample size was not small.
Conclusions
Primiparous age above 30 years was a poor prognostic factor of OS, PFS, and quality of life. Common factors such as age at diagnosis below 40 years, LNM, non-SCC, and larger tumors were confirmed to be poor prognostic factors of OS and PFS, while the protective factors were weight loss, good mental status, and standardized treatment. The quality of life was better in patients with a younger age at diagnosis, active review according to medical orders, and earlier return to society.
Acknowledgments
The authors would like to show their sincere gratitude to all the faculty members in their departments and all the patients and their families involved in this study.
Funding: This study was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.02.70). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the ethics committee at Harbin Medical University in Harbin, China. All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors. Informed consent was obtained from all individual participants included in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Chen WQ, Sun KX, Zheng RS, et al. Report of Cancer Incidence and Mortality in Different Areas of China, 2014. China Cancer 2018;27:1-14.
- Bazaz M, Shahry P, Latifi SM, et al. Cervical Cancer Literacy in Women of Reproductive Age and Its Related Factors. J Cancer Educ 2019;34:82-9. [Crossref] [PubMed]
- Marquina G, Manzano A, Casado A. Targeted Agents in Cervical Cancer: Beyond Bevacizumab. Curr Oncol Rep 2018;20:40. [Crossref] [PubMed]
- Wright JD, Chen L, Tergas AI, et al. Population-level trends in relative survival for cervical cancer. Am J Obstet Gynecol 2015;213:670.e1-7. [Crossref] [PubMed]
- Fernandez A, Sturmberg J, Lukersmith S, et al. Evidence-based medicine: is it a bridge too far? Health Res Policy Syst 2015;13:66. [Crossref] [PubMed]
- Metge CJ. What comes after producing the evidence? The importance of external validity to translating science to practice. Clin Ther 2011;33:578-80. [Crossref] [PubMed]
- Dang A, Kaur K. Comparative effectiveness research and its utility in In-clinic practice. Perspect Clin Res 2016;7:9-14. [Crossref] [PubMed]
- Dang A, Vallish BN. Real world evidence: An Indian perspective. Perspect Clin Res 2016;7:156-60. [Crossref] [PubMed]
- Ariga T, Toita T, Kato S, et al. Treatment outcomes of patients with FIGO Stage I/II uterine cervical cancer treated with definitive radiotherapy: a multi-institutional retrospective research study. J Radiat Res 2015;56:841-48. [Crossref] [PubMed]
- Liu YM, Ni LQ, Wang SS, et al. Outcome and prognostic factors in cervical cancer patients treated with surgery and concurrent chemoradiotherapy: a retrospective study. World J Surg Oncol 2018;16:18-24. [Crossref] [PubMed]
- Harsh KK, Kapoor A. Induction Chemotherapy Followed by Concurrent Chemoradiation in the Management of Different Stages of Cervical Carcinoma: 5-year Retrospective Study. J Obstet Gynaecol India 2016;66:372-78. [Crossref] [PubMed]
- Twu NF, Ou YC, Liao CI, et al. Prognostic factors and adjuvant therapy on survival in early-stage cervical adenocarcinoma/adenosquamous carcinoma after primary radical surgery: A Taiwanese Gynecologic Oncology Group (TGOG) study. Surg Oncol 2016;25:229-35. [Crossref] [PubMed]
- Winer I, Alvarado-Cabrero I, Hassan O, et al. The prognostic significance of histologic type in early stage cervical cancer - A multi-institutional study. Gynecol Oncol 2015;137:474-8. [Crossref] [PubMed]
- Lee KC, Kim HJ, Sung K, et al. The predictive value of tumor size, volume, and markers during radiation therapy in patients with cervical cancer. Int J Gynecol Cancer 2017;27:123-30. [Crossref] [PubMed]
- Koh WJ, Abu-Rustum NR, Bean S, et al. Cervical Cancer, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2019;17:64-84. [Crossref] [PubMed]
- Wang W, Hou X, Yan J, et al. Outcome and toxicity of radical radiotherapy or concurrent Chemoradiotherapy for elderly cervical cancer women. BMC Cancer 2017;17:510. [Crossref] [PubMed]
- Koh WJ, Greer BE, Abu-Rustum NR, et al. Cervical Cancer, Version 2.2015. J Natl Compr Canc Netw 2015;13:395-404. [Crossref] [PubMed]
- Clark LH, Jackson AL, Soo AE, et al. Extremes in body mass index affect overall survival in women with cervical cancer. Gynecol Oncol 2016;141:497-500. [Crossref] [PubMed]
- Pelkofski E, Stine J, Wages NA, et al. Cervical Cancer in Women Aged 35 Years and Younger. Clin Ther 2016;38:459-66. [Crossref] [PubMed]
- Wang J, Wang T, Yang YY, et al. Patient age, tumor appearance and tumor size are risk factors for early recurrence of cervical cancer. Mol Clin Oncol 2015;3:363-6. [Crossref] [PubMed]
- Haile ZT, Kingori C, Chavan B, et al. Association between risky sexual behavior and cervical cancer screening among women in Kenya: a population-based study. J Community Health 2018;43:238-47. [Crossref] [PubMed]
- González D, Suárez EL, Ortiz AP. Cervical Cancer Screening and Sexual Risky Behaviors among a Population of Hispanic Origin. Women’s Health Issues 2015;25:254-61. [Crossref] [PubMed]
- Lahita RG. The effects of sex hormones on the immune system in pregnancy. Am J Reprod Immunol 1992;28:136-7. [Crossref] [PubMed]
- Wuu J, Hellerstein S, Lipworth L, et al. Correlates of pregnancy oestrogen, progesterone and sex hormone-binding globulin in the USA and China. Eur J Cancer Prev 2002;11:283-93. [Crossref] [PubMed]
- World Health Organization: WHO Cancer Control Strategy. 2005.
- Bogani G, Ditto A, Martinelli F, et al. Impact of Blood Transfusions on Survival of Locally Advanced Cervical Cancer Patients Undergoing Neoadjuvant Chemotherapy Plus Radical Surgery. Int J Gynecol Cancer 2017;27:514-22. [Crossref] [PubMed]
- Gargiulo P, Arenare L, Pisano C, et al. Long-Term Toxicity and Quality of Life in Patients Treated for Locally Advanced Cervical Cancer. Oncology 2016;90:29-35. [Crossref] [PubMed]
- Grion RC, Baccaro LF, Vaz AF, et al. Sexual function and quality of life in women with cervical cancer before radiotherapy: A pilot study. Arch Gynecol Obstet 2016;293:879-86. [Crossref] [PubMed]
- Huang HY, Tsai WC, Chou WY, et al. Quality of life of breast and cervical cancer survivors. BMC Womens Health 2017;17:30-41. [Crossref] [PubMed]


