Relationships of hepatitis B virus infection with clinicopathological features in breast cancer and survival outcomes in central China
Introduction
Breast cancer (BC) is the leading cause of cancer-related death in female worldwide, accounting for approximately 24.2% of the cancer incidence and 15% of cancer-specific death among women (1). Despite the lower rates of BC incidence compared with developed countries, the BC rate in China has been double the global rate since the 1990s as a result of the rising socioeconomic status and unique reproductive patterns of this country (2). There is evidence for a link between infectious agents, inflammation, and cancer development. The infectious agents, including bacteria, viruses, and parasites, have been correlated with up to 16% of global cancer incidence (3), with some types of viruses having been researched for their potential connection to BC (4).
Chronic hepatitis B virus (HBV) infection is a significant global health burden, but it is particularly grievous in China. Globally, approximately 2 billion people have had contact with HBV in their lifetime, and 350 million of them are chronic carriers (5). In China, the prevalence of HBV infection is much higher than it is in the United States and Western Europe, affecting 97 million people. Although the HBV immunization program initiated in 1992 has reduced the newborn hepatitis B virus surface antigen (HBsAg) carrier rate from 9.8% to a rate of 7.2% in 2006, chronic HBV carriers remain a severe social health burden to Chinese society (6).
Chronic infection with HBV, a hepadnavirus, causes continuous damage to hepatocytes, leading to the dysfunction of estrogen inactivation. Meanwhile, high levels of free estrogen in the bloodstream can increase the incidence of BC and promote cancer development (7). Research on the molecular level reveals that the X protein of HBV (HBX) is expressed in breast tissue and transactivates the p53 tumor suppressor gene, promoting BC carcinogenesis (8). Moreover, HBX-interacting protein (HBXIP) interacts with HBX and participates in lipid metabolism, leading to an inverse effect on BC metastasis (9). These phenomena suggest that HBV infection has a strong correlation with BC. However, few studies had been conducted that have evaluated the effect of HBV infection on BC. In this article, we performed a retrospective case-control study for verifying the clinicopathological features and outcomes between chronic HBV carriers and people who have never had contact with HBV among BC patients.
Methods
Patient population
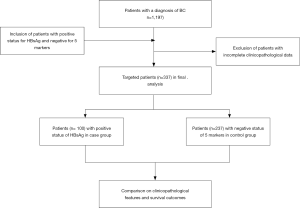
Female patients with primary BC treated at the Breast and Thyroid Center, Renmin Hospital, Wuhan University between January 2013 and December 2017 were included in this study. The exclusion criteria were metastatic BC (stage IV) at initial diagnosis and incomplete clinicopathological data. In China, for each individual prepared for surgical treatment, all routine serological tests for pathogens, including HBV, hepatitis C virus (HCV), syphilis, and human immunodeficiency virus (HIV), must be carried out during hospitalization. In this study, 5 markers of HBV [HBsAg, hepatitis B “e” antigen (HBeAg), anti-HBV surface antibody (anti-HBs), anti-HBV “e” antibody (anti-HBe), and anti-HBV core antibody (anti-HBc)] were collected from the electronic medical record (EMR) system. Among 1,191 consecutive patients, we started a case-control study and found that 100 (8.4%) individuals were infected by HBV and had positive HBsAg status (case group), while 237 (19.9%) had never come into contact with HBV and were negative for HBsAg, HBeAg, anti-HBs, anti-HBe, and anti-HBc (control group). The flow diagram for our case-control study is shown in Figure 1.
Follow-up
The follow-up duration was counted from the date of initial diagnosis to our last contact with the patients or patients’ death. Patients’ disease-free survival (DFS) rates were recorded as the period between tumor diagnosis and local-regional relapse or distant metastasis. The follow-up deadline was March 2019.
Data collection
The clinicopathological features included the assessment of age at diagnosis, menopausal status, primary tumor size, histological grade, lymph node invasion, estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (Her-2), and percentage of Ki-67. These were all obtained from the EMR and primary pathology reports. Histological type and grade were based on the 2012 World Health Organization (WHO) classification of breast neoplasm. Tumor staging of BC followed the seventh American Joint Committee on Cancer (AJCC) criteria from 2017. Immunohistochemical staining followed the guideline for the detection of ER and PR immunohistochemistry in BC and BC Her-2 guideline (2014 edition). The cutoff for ER and PR positivity was 1%. Her-2(0) and Her-2(1+) were considered to be a negative status for Her-2 overexpression, Her-2(3+) was used to indicate overexpression, and Her-2(2+) required fluorescence in situ hybridization (FISH) testing for the final determination. All the pathological diagnoses were viewed by 2 or more pathologists in Renmin Hospital, Wuhan University.
Statistics
Baseline demographic and tumor clinicopathological characteristics were compared between the case and control group using chi-square tests for frequencies. DFS estimates were performed using Kaplan-Meier survival analyses, and the log-rank test was used to examine the difference between the case and control groups. Univariate and multivariate analyses assessed the effect of the clinicopathological parameters on the outcomes using the Cox proportional hazard models, and hazard ratios (HRs) and 95% confidence intervals (95% CIs) were computed. Variable estimates were considered significant with a P value <0.05. Data were analyzed using IBM SPSS Statistics software version 22.0.
Results
A total of 1,191 patients were diagnosed with primary BC between 2012 and 2017; among these women, 100 (8.4%) had a positive HBsAg status (case group), while 237 (19.9%) were negative for HBV infection and had a negative status for HBsAg, HBeAg, anti-HBs, anti-HBe, and anti-HBc (control group). The comparison of the clinicopathological characteristics in BC patients between the case and control groups is given in Table 1. Patients in the case group tended to have smaller tumors (T <2 cm) compared with the control group (53.0% vs. 65.8%, P<0.05). The ratio of histological grade 3 was significantly higher in the case group compared with that in the control group (55.0% vs. 37.6%, P<0.01). The case group had slightly lower results for age at diagnosis and lymph node invasion, but the difference was not statistically significant. Other features, like menopausal status, metastasis, ER, PR, Her-2, and percentage of Ki-67, exhibited no significant difference between the case and control group.
Table 1
| Variable | Case (n=100) | Control (n=237) | P value |
|---|---|---|---|
| Age | 0.679 | ||
| ≤35 | 4 (4%) | 12 (5.1%) | |
| >35 | 96 (96%) | 225 (94.9%) | |
| Menopausal status | 0.102 | ||
| Premenopausal | 57 (57%) | 112 (47.3%) | |
| Postmenopausal | 43 (43%) | 225 (52.7%) | |
| Tumor size (cm) | 0.027* | ||
| ≤2 | 47 (47%) | 81 (34.2%) | |
| >2 | 53 (53%) | 156 (65.8%) | |
| Histological grade | 0.003** | ||
| G1 + G2 | 45 (45%) | 148 (62.4%) | |
| G3 | 55 (55%) | 89 (37.6%) | |
| Lymph-node status | 0.130 | ||
| Negative | 65 (65%) | 133 (56.1%) | |
| Positive | 35 (35%) | 104 (43.9%) | |
| ER status | 0.711 | ||
| Negative | 35 (35%) | 88 (37.1%) | |
| Positive | 65 (65%) | 149 (62.9%) | |
| PR status | 0.945 | ||
| Negative | 46 (46%) | 110 (46.4%) | |
| Positive | 54 (54%) | 127 (53.6%) | |
| Her-2/neu status | 0.354 | ||
| Negative | 71 (71%) | 156 (65.8%) | |
| Positive | 29 (29%) | 81 (34.2%) | |
| Ki-67 (%) | 0.413 | ||
| <20 | 30 (30%) | 82 (34.6%) | |
| ≥20 | 70 (70%) | 155 (65.4%) |
Case group: positive status for HBsAg; control group: negative status for HBsAg. *, P<0.05; **, P<0.01. HBeAg, anti-HBs; anti-HBe, anti-HBc.
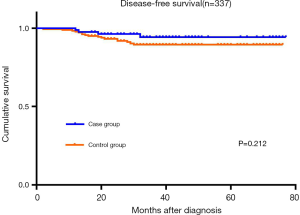
With a median follow-up duration of 34.5 months (2–76 months), we saw 21 cases of tumor recurrence in the available patients: 4 in the case group and 17 in the control group. The 3-year DFS rates were 94.3% in the case group and 89.4% in the control group (P=0.227; Figure 2).
In univariate analysis, nodal status (HR =4.789, P=0.002), ER (HR =0.212, P=0.001), PR (HR =0.317, P=0.017), and Her-2 (HR =2.673, P=0.026) showed significant differences in terms of the patients’ DFS, while there was no difference related to HBV infection, age, and ki-67 (P>0.05). For multivariate analysis, nodal status (HR =5.033, P=0.003) and ER status (HR =0.216, P=0.023) were both independent prognostic risk factors of DFS (Table 2).
Table 2
| Variable | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| HBV infection | 0.518 (0.174–1.538) | 0.236 | 0.886 (0.283–2.772) | 0.835 | |
| Age | 0.750 (0.100–5.602) | 0.779 | 1.514 (0.199–11.522) | 0.689 | |
| T stage | 2.899 (0.975–8.616) | 0.056 | 1.970 (0.632–6.140) | 0.243 | |
| Nodal status | 4.789 (1.754–13.075) | 0.002 | 5.033 (1.734–14.612) | 0.003 | |
| ER status | 0.212 (0.082–0.546) | 0.001 | 0.216 (0.057–0.808) | 0.023 | |
| PR status | 0.317 (0.123–0.816) | 0.017 | 0.743 (0.194–2.843) | 0.664 | |
| Her-2 status | 2.673 (1.126–6.345) | 0.026 | 1.367 (0.531–3.523) | 0.517 | |
| Ki-67 | 1.485 (0.576–3.827) | 0.413 | 0.533 (0.172–1.652) | 0.275 | |
HBV, hepatitis B virus; HR, hazard ratio; 95% CI, 95% confidence interval.
Discussion
Recent studies have revealed that infectious viral agents, such as human papillomavirus (HPV), human cytomegalovirus (HCMV), Epstein-Barr virus (EBV), and simian virus 40 (SV40) (10-13), are related to the development of BC. Their virus-associated gene sequences have been detected in BC tissue and para-carcinoma normal breast tissue, suggesting a relationship between viral infection and the development of BC exists. About 2 billion people are infected with HBV, and HBV infection has become an enormous health burden worldwide. China was a high endemic area of hepatitis B in the last century. After the HBV seroepidemiological surveys were conducted in 1992 and 2006 throughout the country, China initiated a nationwide HBV immunization program and provided free HBV vaccination for neonates, leading to a decline of HBsAg prevalence from 9.75% to 7.18% (14). In 2014, a serological survey conducted nationwide showed a lower than 6% prevalence of HBsAg carriers (15). Of the 1,191 patients in our study, 8.4% had positive HBsAg status. The incidence rate of HBsAg was higher than that in 2014; meanwhile, the prevalence of HBsAg was higher in males than females in China, indicating a correlation between HBV infection and BC.
In our study, the patients in the case group, who were positive for HBsAg, exhibited a smaller tumor size (T ≤2 cm) at diagnosis than the control group (P=0.027), but there was a higher ratio for the percentage of histological grade 3 in the case group (P=0.003). These points reveal an earlier stage and more invasive status in patients positive for HBsAg. Patients in the case group were slightly younger in age and exhibited less lymph node invasion, although these results were not statistically significant. One explanation for these results could be that HBV-infected patients paid greater attention to their health condition. Alternatively, HBX and HBXIP might have contributed to the higher level of histological grade and the more aggressive presence in the lymph nodes. In fundamental studies, HBX was associated with the overexpression of cyclin D1 and was found to promote the development of BC in transgenic mice (16). As a crucial component regulator complex binding to the C terminus of HBX expressed in BC cells and clinical cancer tissue, HBXIP upregulated methyltransferase-like 3 (METTL3), which in turn, promoted the expression of HBXIP, thereby accelerating the progress of BC (17).
According to the survival analysis, our study showed that the prognostic trend in the case group was somewhat better than that in the control group (Figure 2). Although there was no statistically significant difference between the case and control groups (P=0.212), the patients with HBsAg positivity had a longer DFS without tumor relapse and metastasis. The analysis results seemed to match the clinicopathological features in tumor size, which means that patients with chronic HBV infection had an earlier stage of the primary tumor that led to better outcomes. These results may have emerged due to the small size of the patient cohort and a population selection bias. In contrast, patients with HBV infection had better compliance with standardized treatment for BC and decent feedback for their attendance. Univariate analysis showed the nodal status and ER had a relationship with patients’ DFS in the case and control groups; multivariate analysis showed that nodal status and ER status were independent prognostic risk factors in DFS. Negative lymph node involvement and positive ER status were associated with better DFS, and thus, our study suggests that DFS may benefit from early surgical treatment and proper endocrine therapy. Similar research has revealed that lymph node involvement and hormone receptor status were independent prognostic factors of survival outcomes (18).
There have been several studies on the relationship between HBV infection and the prevalence of BC. In Taiwan, a large cohort retrospective case-control study covering 1,958 patients investigated whether BC was associated with chronic hepatitis virus infection, and multivariate logistic regression showed that BC patients aged <50 years were at a 2-fold greater risk than the control group (19). A multicenter study in Korea enrolled more than 200,000 patients with non-hepatocellular malignancy from 2007 to 2014, and the researchers found that 12,487 patients with primary BC exhibited a significant association with HBV infection (adjusted odds ratio 1.16, 95% CI: 1.02–1.32), suggesting that HBV screening would be necessary for the future (20). Recently, a study conducted in China explored the relationship between HBV infection and extrahepatic carcinoma in younger people; it was found that BC patients with HBsAg positivity had a younger mean age at diagnosis, while the age subgroup of 0–39 years had a higher proportion of HBsAg-positive patients (21). Finally, a study performed in an elderly American population revealed an inverse association between HBV infection and BC (22); this is an interesting result as it seems in line with our study in terms of the better survival outcome. However, the studies above did not investigate the clinicopathological features or real-world survival outcomes; a deeper exploration of our research should be conducted in future studies.
Some limitations to the present study should also be addressed. First, it was a single-center study, and the sample size was not large. In addition, it might have been affected by selection bias, and the short duration of events we were interested in might have influenced the results. We did not observe a difference in the patients’ DFS; however, increased sample size or prolonged follow-up could perhaps yield a significant difference in DFS and even overall survival (OS) among BC patients with HBV infection.
In conclusion, BC patients in central China have a higher incidence rate of HBV infection than the general population. BC patients with chronic HBV infection exhibit an earlier T stage and worse histological grade, although their local and distal recurrence conditions are more positive.
Acknowledgments
We sincerely thank the patients who participated in this study and the medical staff in the Breast and Thyroid Center, Renmin Hospital of Wuhan University.
Funding: This work was partially supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.03.15). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The patient healthcare record data used for this work was retrospective in nature and approved by Renmin Hospital of Wuhan University (No. 20133655893). Informed consent was obtained from all individual participants included in the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Fan L, Strasser-Weippl K, Li JJ, et al. Breast cancer in China. Lancet Oncol 2014;15:e279-89. [Crossref] [PubMed]
- Chan SL, Wong VW, Qin S, et al. Infection and Cancer: The Case of Hepatitis B. J Clin Oncol 2016;34:83-90. [Crossref] [PubMed]
- Buehring GC, Shen H, Schwartz DA, et al. Bovine leukemia virus linked to breast cancer in Australian women and identified before breast cancer development. PLoS One 2017;12:e0179367. [Crossref] [PubMed]
- Islami F, Chen W, Yu XQ, et al. Cancer deaths and cases attributable to lifestyle factors and infections in China, 2013. Ann Oncol 2017;28:2567-74. [Crossref] [PubMed]
- Li T, Mello-Thoms C, Brennan PC. Descriptive epidemiology of breast cancer in China: incidence, mortality, survival and prevalence. Breast Cancer Res Treat 2016;159:395-406. [Crossref] [PubMed]
- Fentiman IS. The endocrinology of male breast cancer. Endocr Relat Cancer 2018;25:R365-R73. [Crossref] [PubMed]
- Adhikari VP, Lu LJ, Kong LQ. Does hepatitis B virus infection cause breast cancer? Chin Clin Oncol 2016;5:81. [Crossref] [PubMed]
- Zhao Y, Li H, Zhang Y, et al. Oncoprotein HBXIP Modulates Abnormal Lipid Metabolism and Growth of Breast Cancer Cells by Activating the LXRs/SREBP-1c/FAS Signaling Cascade. Cancer Res 2016;76:4696-707. [Crossref] [PubMed]
- El-Naby NEH, Hassan Mohamed H, Mohamed Goda A, et al. Epstein-Barr virus infection and breast invasive ductal carcinoma in Egyptian women: A single center experience. J Egypt Natl Canc Inst 2017;29:77-82. [Crossref] [PubMed]
- Kouloura A, Nicolaidou E, Misitzis I, et al. HPV infection and breast cancer. Results of a microarray approach. Breast 2018;40:165-9. [Crossref] [PubMed]
- Cui J, Wang Q, Wang HB, et al. Protein and DNA evidences of HCMV infection in primary breast cancer tissues and metastatic sentinel lymph nodes. Cancer Biomark 2018;21:769-80. [Crossref] [PubMed]
- Mazzoni E, Bononi I, Benassi MS, et al. Serum Antibodies Against Simian Virus 40 Large T Antigen, the Viral Oncoprotein, in Osteosarcoma Patients. Front Cell Dev Biol 2018;6:64. [Crossref] [PubMed]
- Wang FS, Fan JG, Zhang Z, et al. The global burden of liver disease: the major impact of China. Hepatology 2014;60:2099-108. [Crossref] [PubMed]
- Hu Y. Seroepidemiology of hepatitis B virus infection in children 12 years after China's expanded program on immunization in Qamdo, Tibet. J Public Health Policy 2018;39:446-53. [Crossref] [PubMed]
- Wang G, Gormley M, Qiao J, et al. Cyclin D1-mediated microRNA expression signature predicts breast cancer outcome. Theranostics 2018;8:2251-63. [Crossref] [PubMed]
- Cai X, Wang X, Cao C, et al. HBXIP-elevated methyltransferase METTL3 promotes the progression of breast cancer via inhibiting tumor suppressor let-7g. Cancer Lett 2018;415:11-9. [Crossref] [PubMed]
- Dai DN, Li Y, Chen B, et al. Elevated expression of CST1 promotes breast cancer progression and predicts a poor prognosis. J Mol Med (Berl) 2017;95:873-86. [Crossref] [PubMed]
- Su FH, Chang SN, Chen PC, et al. Association between chronic viral hepatitis infection and breast cancer risk: a nationwide population-based case-control study. BMC Cancer 2011;11:495. [Crossref] [PubMed]
- An J, Kim JW, Shim JH, et al. Chronic hepatitis B infection and non-hepatocellular cancers: A hospital registry-based, case-control study. PLoS One 2018;13:e0193232. [Crossref] [PubMed]
- Lu T, Yang Q, Li M, et al. HBV infection and extra-hepatic cancers in adolescents and 20s: A retrospective study in China. Cancer Epidemiol 2018;55:149-55. [Crossref] [PubMed]
- Mahale P, Torres HA, Kramer JR, et al. Hepatitis C virus infection and the risk of cancer among elderly US adults: A registry-based case-control study. Cancer 2017;123:1202-11. [Crossref] [PubMed]