Hereditary multiple exostoses complicated with lung cancer with cough as the first symptom: a case report
Introduction
Hereditary multiple exostoses (HME) is a rare hereditary musculoskeletal disorder, which is a kind of benign bone tumors. It is mainly characterized by the exostosis formed in the perichondrium and covered by the cartilage cap (1). It mostly involves the long bone, rib, scapula, pelvis and vertebral body. Studies have shown that HME is related to the deletion mutation of exorosin-1 (EXT1) and exorosin-2 (EXT2) (2,3). The coding products of EXT1 and EXT2 genes can form heparan sulfate, further form heparan sulfate proteoglycan (4,5), and maintain the proliferation and differentiation of normal chondrocytes (6). About 0.5–2% of HME will malignant into chondrosarcoma or osteosarcoma (7,8). When the cartilage cap continues to grow or the patient has persistent pain, it may indicate that the tumor has malignant transformation (9). The cases of HME with lung cancer are rare and only one case reported in Russia in 1981 was found (10). Herein, we present the following case in accordance with the CARE Guideline (11).
Case presentation
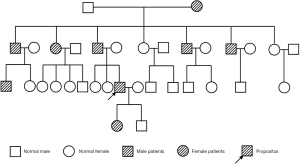
A 33-year-old male patient with a history of HME was admitted to local hospital due to cough and chest pain for almost half a year. The local hospital initially gave antitussive and anti-infective treatment, but the symptoms still recurred. Chest CT scan imaging showed that an occupying lesion in the right middle lobe and enlarged lymph nodes in the mediastinum of the right hilar lung. The endoscopic bronchoscopy indicated that there was stenosis of the right middle lobar bronchi under external pressure. The tumor marker suggested CA125 was 81.75 U/mL. Therefore, the patient was recommended to transfer for further diagnosis and admitted to our hospital on August 6, 2019. He was operated on in 2001 because of the compression by the exostoses of the lateral left knee. His paternal family had a history of HME (Figure 1). The patient denied other chronic diseases, smoking or drinking history.
The physical examination showed that the body temperature was 36.6 °C, the heart rate was 70 beats/min, the respiratory rate was 14 breaths/min, and the blood pressure was 117/81 mmHg. The height of the patient was 156 cm, and the weight was 63 kg. Lung examination showed no thoracic deformity and no tenderness. The auscultation of both lungs was clear. The left acromioclavicular joint, the left shoulder joint, the right elbow joint, the bilateral knee joint and the left ankle joint could be seen with different sizes of protuberance. The fifth fingers and the fourth toes were slightly short. No obvious abnormality was found in the extremity movement and strength.
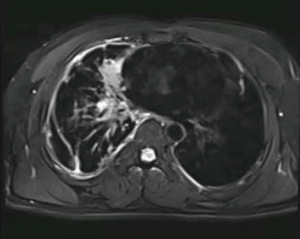
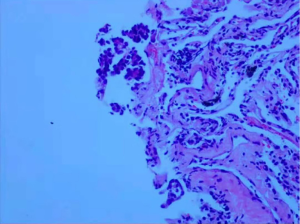
Laboratory data indicated that erythrocyte sedimentation rate (ESR) was 25 mm/h and the blood potassium level was 3.34 mmol/L. The tuberculosis antibody was weak positive and T-SPOT was negative. The rest of the laboratory data was unremarkable. CT of the thorax in our hospital was similar to that before. A 3.5×2.4 cm2 irregular lesion showed a high density image in the middle lobe of the right lung and multiple patchy shadows scattered under the right lung pleura with local pleural thickness and adhesion, small amounts of pleural effusion. Multiple enlarged lymph nodes were found in mediastinum. There were multiple cauliflower-like exostosis on both ribs and scapula (Figure 2). The magnetic resonance imaging (MRI) result was consistent with CT result (Figure 3). In order to find out the occupying lesion in the right upper lobe, CT-guided percutaneous lung biopsy was performed. Pathology results showed that there were lung tissue arranged in cords with few allotypic cells (Figure 4). Immunohistochemistry results showed that few allotypic cells pointed out CK7(+), TTF-1(+), WT-1(−), Calretinin(−), CK5/6(−), D2-40(−). Combined with HE sections, this case was consistent with infiltrative adenocarcinoma. To determine the clinical stage of tumor, PET/CT examination was performed, which suggested that right lung cancer with right intrapulmonary and right pleura metastasis. The irregular edges of ribs and neck of femur on both sides were considered as multiple exostoses. Molecular pathologic analysis results showed no EGFR mutations. According to the results of PET/CT and pathology, the diagnosis of right lung adenocarcinoma, T3N2M1a, stage IVA was considered.
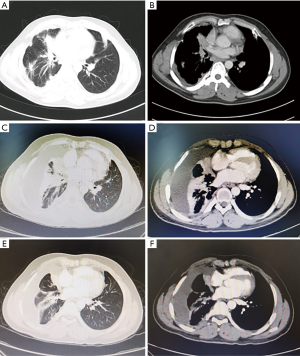
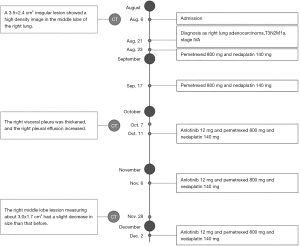
The patient was started on pemetrexed 800 mg combined with nedaplatin 140 mg given every 3 weeks for 2 cycles on August 23 and September 17. After the second cycle, reexamination of chest CT showed that the right lung lesions were similar to the former, the right visceral pleura was thickened, and the right pleural effusion increased (Figure 2). Considering that the increase of pleural fluid was caused by pleural metastasis, we adjusted the treatment plan. The patient was added anlotinib 12 mg combined with pemetrexed 800 mg and nedaplatin 140 mg for additional 2 cycles on October 11 and November 6. A subsequent chest CT showed that the right middle lobe lesion measuring about 3.0×1.7 cm2 had a slight decrease in size than that before (Figure 2). As a result, an additional 1 cycle of treatment was given on December 2 (for details see Figure 5). The patient is still under active treatment and further follow-up is in progress.
Discussion
HME with primary lung cancer is rare. After searching the related researches, only one case reported in Russia in 1981 was found (10). This case admitted in our hospital was a young man with cough as the first symptom. He had a paternal family history of HME. The main clinical symptoms of HME in the rest of the family were mostly caused by tumor compression, most of which did not cause dysfunction. The family history of malignant tumor was denied. According to the results of PET/CT, pathology and immunohistochemistry, right lung adenocarcinoma, T3N2M1a, stage IVA could be diagnosed finally. Due to the limited economic conditions of patients, we were not able to complete all molecular pathologic analysis. However, this case provides new thinking for our future diagnosis. When we meet patients with such multiple exostoses and lung occupying lesions, we need to think about many possibilities of the disease from various perspectives, such as multiple exostoses with primary lung cancer, malignant transformation of osteochondroma into chondrosarcoma complicated with lung metastasis, primary lung cancer with bone metastasis and hypertrophic pulmonary osteoarthropathy (HPOA), etc.
In the case of multiple exostoses with primary lung cancer, the diagnosis of primary lung cancer can be determined by combining imaging examination, pathological analysis, molecular pathologic analysis and other methods. In terms of the choice of treatment plan, this patient chose the conventional chemotherapy plan for non-small cell lung cancer, namely pemetrexed combined with platinum complexes. At present, the clinical treatment of HME is mainly surgery, including exostoses resections and orthopedic surgery for patients with obvious compression symptoms. The patient had no obvious symptoms this hospitalization, so multiple exostoses were not treated. Some researches have shown that estrogen is related to the occurrence, development and prognosis of lung cancer. β-estradiol can be detected in high concentration in non-small cell lung cancer tissues (12), which can stimulate tumor growth (13) and induce malignant transformation of normal tissues (14). Because chondrosarcoma has estrogen receptor, it has been proved to be sensitive to estrogen (15,16). The use of antiestrogen drugs may stimulate the growth of chondrosarcoma, thus increasing the risk of malignant transformation of HME into chondrosarcoma (17). Therefore, this kind of patients are not suitable for anti-estrogen treatment. Whether estrogen is the correlation point between these two diseases, and whether HME can increase the risk of lung cancer, many mysteries still need to be further explored.
About 0.5–2% of HME will turn into low-grade and highly differentiated peripheral chondrosarcoma (18). Compared with primary chondrosarcoma, secondary chondrosarcoma has less metastasis and better prognosis. After successful surgical treatment, patients may achieve long-term disease-free survival (19). About 10% of chondrosarcomas are dedifferentiated (18). It has been shown that peripheral dedifferentiated chondrosarcoma accounts for 3.86% (20) and 5.5% (21) of all dedifferentiated chondrosarcomas. It can be secondary to benign osteochondroma, with strong invasion, poor prognosis and low survival rate (22), in which the lung is the most common site of metastasis (19). If considering the malignant transformation of osteochondroma into chondrosarcoma complicated with pulmonary metastasis, the clinical manifestations are usually sudden swelling and persistent pain of the focus of osteochondroma, and the pulmonary symptoms generally appear later. In this case, cough was the first manifestation, and there was no symptom of bone compression. Therefore, the possibility could be excluded.
Bone metastasis is one of the main metastasis sites of lung cancer (23), the incidence is about 10–15% (24), most of which are osteolytic lesions (25). It often accompanied by bone pain and bone related events, such as pathological fracture, spinal cord compression (25), Bone metastasis is the first symptom in many patients. At present, radionuclide bone scan and PET/CT are the main screening methods. The PET/CT findings of this patient showed that the edges of ribs and the femoral neck at both sides were irregular, and there was no increase in FDG metabolism. Multiple chondroma may be considered. So, bone metastasis of lung cancer was excluded. However, for the high-risk patients who have a history of osteochondroma complicated with lung cancer and have new symptoms of bone pain, we still need to be aware of the possibility of bone metastasis.
HPOA is a syndrome characterized by digital clubbing, arthritis, and periostitis, which caused by lung disease (26). Its clinical characteristics are bilateral symmetric periosteal hyperplasia and new bone formation, which mainly affects long bone. Adenocarcinoma is common. The main pathogenesis may be related to abnormal secretion of growth hormone, autoimmune response, blood circulation disorders and so on (27). Joint pains usually precede pulmonary symptoms, so it is easy to missed diagnosis and misdiagnosis. When the primary tumor is treated with radiotherapy (28), chemotherapy (29) and surgical resection (30), the symptoms of bones and joints will be improved accordingly. The patient had no new symptoms of bone and joint, so it was excluded.
To sum up, the clinical cases of HME with primary lung cancer are rare. The diagnosis depends on the comprehensive judgment of clinical manifestations, imaging means and pathological examination. In the process of clinical diagnosis and treatment, it is possible to make more accurate diagnosis and targeted treatment by considering various clinical aspects. Whether HME is a risk factor for lung cancer and its related pathogenesis remains to be further studied.
Acknowledgments
Funding: This research was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.02.22). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pacifici M. Hereditary Multiple Exostoses: New Insights into Pathogenesis, Clinical Complications, and Potential Treatments. Curr Osteoporos Rep 2017;15:142-52. [Crossref] [PubMed]
- Cook A, Raskind W, Blanton SH, et al. Genetic heterogeneity in families with hereditary multiple exostoses. Am J Hum Genet 1993;53:71-9. [PubMed]
- Ahn J, Ludecke HJ, Lindow S, et al. Cloning of the putative tumour suppressor gene for hereditary multiple exostoses (EXT1). Nat Genet 1995;11:137-43. [Crossref] [PubMed]
- Lindahl U. Heparan sulfate-protein interactions--a concept for drug design? Thromb Haemost 2007;98:109-15. [Crossref] [PubMed]
- Li JP. Heparin, heparan sulfate and heparanase in cancer: remedy for metastasis? Anticancer Agents Med Chem 2008;8:64-76. [Crossref] [PubMed]
- Clément A, Wiweger M, von der Hardt S, et al. Regulation of zebrafish skeletogenesis by ext2/dackel and papst1/pinscher. PLoS Genet 2008;4:e1000136. [Crossref] [PubMed]
- Wicklund CL, Pauli RM, Johnston D, et al. Natural history study of hereditary multiple exostoses. Am J Med Genet 1995;55:43-6. [Crossref] [PubMed]
- Legeai-Mallet L, Munnich A, Maroteaux P, et al. Incomplete penetrance and expressivity skewing in hereditary multiple exostoses. Clin Genet 1997;52:12-6. [Crossref] [PubMed]
- Huvos AG, Marcove RC. Chondrosarcoma in the young. A clinicopathologic analysis of 79 patients younger than 21 years of age. Am J Surg Pathol 1987;11:930-42. [Crossref] [PubMed]
- Chudina AP, Akulenko LV, Prokopenko VD. Combination of multiple exostoses and lung cancer in 1 family. Vopr Onkol 1981;27:81-3. [PubMed]
- Riley DS, Barber MS, Kienle GS, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol 2017;89:218-35. [Crossref] [PubMed]
- Niikawa H, Suzuki T, Miki Y, et al. Intratumoral estrogens and estrogen receptors in human non-small cell lung carcinoma. Clin Cancer Res 2008;14:4417-26. [Crossref] [PubMed]
- Tang H, Liao Y, Xu L, et al. Estrogen and insulin-like growth factor 1 synergistically promote the development of lung adenocarcinoma in mice. Int J Cancer 2013;133:2473-82. [Crossref] [PubMed]
- Liu W, Konduri SD, Bansal S, et al. Estrogen receptor-alpha binds p53 tumor suppressor protein directly and represses its function. J Biol Chem 2006;281:9837-40. [Crossref] [PubMed]
- Mackintosh D, Mason RM. Pharmacological actions of 17 beta-oestradiol on articular cartilage chondrocytes and chondrosarcoma chondrocytes in the absence of oestrogen receptors. Biochim Biophys Acta 1988;964:295-302. [Crossref] [PubMed]
- Mummaneni PV, Rosenberg WS. Spinal chondrosarcoma following adenocarcinoma of the breast: case report. Surg Neurol 2000;53:580-2. [Crossref] [PubMed]
- Pandya NK, Auerbach JD, Baldwin K, et al. Spinal cord compression in a patient with multiple hereditary exostoses caused by breast adenocarcinoma metastatic to osteochondromas of the spine: case report. Spine (Phila Pa 1976) 2006;31:E920-4. [Crossref] [PubMed]
- Rozeman LB, de Bruijn IH, Bacchini P, et al. Dedifferentiated peripheral chondrosarcomas: regulation of EXT-downstream molecules and differentiation-related genes. Mod Pathol 2009;22:1489-98. [Crossref] [PubMed]
- Ahmed AR, Tan TS, Unni KK, et al. Secondary chondrosarcoma in osteochondroma: report of 107 patients. Clin Orthop Relat Res 2003;193-206. [Crossref] [PubMed]
- Case 4—Dedifferentiated Chondrosarcomas. Nascimento AG. USCAP 2006 Annual Meeting, Bone & Soft Tissue Pathology; 2006.
- Staals EL, Bacchini P, Mercuri M, et al. Dedifferentiated chondrosarcomas arising in preexisting osteochondromas. J Bone Joint Surg Am 2007;89:987-93. [Crossref] [PubMed]
- Akahane T, Shimizu T, Isobe K, et al. Dedifferentiated chondrosarcoma arising in a solitary osteochondroma with leiomyosarcomatous component: a case report. Arch Orthop Trauma Surg 2008;128:951-3. [Crossref] [PubMed]
- Li S, Peng Y, Weinhandl ED, et al. Estimated number of prevalent cases of metastatic bone disease in the US adult population. Clin Epidemiol 2012;4:87-93. [PubMed]
- Hernandez RK, Wade SW, Reich A, et al. Incidence of bone metastases in patients with solid tumors: analysis of oncology electronic medical records in the United States. BMC Cancer 2018;18:44. [Crossref] [PubMed]
- Tanvetyanon T, Hines E Jr. Long-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with nonsmall cell lung carcinoma and other solid tumors. Cancer 2005;103:1756-7; author reply 1757-8.
- Katsurada N, Tachihara M, Jimbo N, et al. Successful Treatment of ROS1-rearranged Lung Cancer Complicated by Hypertrophic Pulmonary Osteoarthropathy with Crizotinib Therapy. Intern Med 2019;58:1467-71. [Crossref] [PubMed]
- Shih WJ. Pulmonary hypertrophic osteoarthropathy and its resolution. Semin Nucl Med 2004;34:159-63. [Crossref] [PubMed]
- Campeau RJ, Rosales OR, Garcia OM, et al. Resolution of hypertrophic pulmonary osteoarthropathy after radiotherapy in the absence of lung tumor response. Clin Nucl Med 1989;14:453-5. [Crossref] [PubMed]
- Dalgleish AG. Hypertrophic pulmonary osteoarthropathy: response to chemotherapy without documented tumour response. Aust N Z J Med 1983;13:513-6. [Crossref] [PubMed]
- Vasudevan CP, Suppiah P, Udoshi MB, et al. Reversible autonomic neuropathy and hypertrophic osteoarthropathy in a patient with bronchogenic carcinoma. Chest 1981;79:479-81. [Crossref] [PubMed]