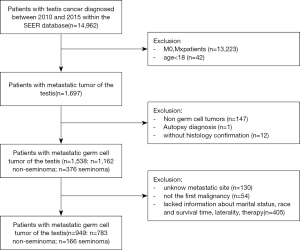
Development and validation of a nomogram to predict survival in patients with metastatic testicular germ cell tumors
Introduction
Testicular germ cell tumors (TGCTs) are the most common solid tumors in men between the ages of 20 and 34 years (1,2), and their incidence has been steadily increasing over the last 60 years (3,4). In the United States (US), an estimated 9,560 new patients with testicular cancer will be diagnosed in 2019, resulting in 410 deaths (2). Poor outcomes in patients with TGCTs are driven primarily by distant metastatic involvement (5). The most common sites of metastatic TGCTs (mTGCTs) include the lymph nodes and lungs (6). Sometimes, distant metastatic sites such as the liver, bone, and brain may be involved (6-9).
In the field of medicine, patients with their medical providers are faced with making multiple decisions based on the estimated probability of a particular event occurring in the future (10). Generally, the American Joint Committee on Cancer tumor–node–metastasis (TNM) staging system and the International Germ Cell Consensus Classification Group (IGCCCG) classification are strongly related to survival; however, different outcomes have also been noted in patients at the same stage. For this reason, a more accurate method of predicting individualized survival outcomes in patients with mTGCTs is required and use of a nomogram is a suitable method for this purpose. Nomograms have been widely used to facilitate the diagnosis and prognosis of diseases (11-14). However, as far as we know, there are no predictive nomograms for patients with mTGCTs. Therefore, in this study, we developed a nomogram to predict survival in patients with mTGCTs using data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program. Our results may provide additional information to medical providers and patients to assist in the decision-making process.
Methods
Our data were obtained from the National Cancer Institute’s SEER program, which covers approximately 28% of the U.S. population. Patients diagnosed between 2010 and 2015 were included in the study because the metastatic site code was only available from 2010. We used the International Classification of Diseases ninth edition codes to identify seminomatous germ cell tumors (SGCTs; 9061–9063) and non-SGCTs (NSGCTs; 9064, 9070–9071, 9080–9085, 9100–9102). Other inclusion criteria included (I) age 18 years or older and diagnosis of primary testicular germ cell cancer; (II) definite distant lymph node, lung, liver, brain and bone metastases; (III) testicular cancer was the first of multiple primaries; (IV) information about cancer-specific survival (CSS) and survival months was available; (V) diagnosis by histologic confirmation. Patients diagnosed from only clinical presentation, radiography, or autopsy were excluded.
Statistical analysis
Continuous variables such as age are presented as means and standard deviations (SDs) and categorical variables such as race are presented as counts and percentages. Survival rates were calculated using Kaplan-Meier curves, and the log-rank test was computed to compare the curves. We used univariate analysis to identify potential risk factors. After the factors were selected, multivariate analyses were performed to select the optimal model. The risk factors considered in the model are those that were considered to be significantly associated with mTGCTs. Harrell's concordance index (C-index), the area under the receiver-operating characteristic curve (AUC) as well as calibration plots were used to assess the performance of the model. All statistical tests were 2-sided, and the significance level was P<0.05. Data were analyzed using the statistical package R (the R foundation; http://www.r-project.org;version3.4.3).
Results
Characteristics of the study patients
A total of 949 patients were included in the analysis according to the aforementioned criteria (Figure 1). The demographic and clinicopathological characteristics of the study patients are presented in Table 1, and the distribution of patients was roughly uniform from 2010 to 2015.
Table 1
| Variable | Total (n=949) | SGCT (n=166) | NSGCT (n=783) | P value |
|---|---|---|---|---|
| Year of diagnosis, No. (%) | 0.2200 | |||
| 2010 | 156 (16.44) | 25 (15.06) | 131 (16.73) | |
| 2011 | 159 (16.75) | 38 (22.89) | 121 (15.45) | |
| 2012 | 148 (15.60) | 25 (15.06) | 123 (15.71) | |
| 2013 | 189 (19.92) | 33 (19.88) | 156 (19.92) | |
| 2014 | 149 (15.70) | 26 (15.66) | 123 (15.71) | |
| 2015 | 148 (15.60) | 19 (11.45) | 129 (16.48) | |
| Age at diagnosis, median (IQR), year | 30 (24–40) | 40 (32–50) | 29 (24–36) | <0.001*** |
| Laterality | 0.8800 | |||
| Left | 458 (48.26) | 81 (48.80) | 377 (48.15) | |
| Right | 491 (51.74) | 85 (51.20) | 406 (51.85) | |
| Lymphovascular invasion, No. (%) | <0.001*** | |||
| Absent | 511 (53.85) | 109 (65.66) | 402 (51.34) | |
| Present | 438 (46.15) | 57 (34.34) | 381 (48.66) | |
| Surgery reg/dis, No. (%) | 0.1100 | |||
| Yes | 95 (10.01) | 11 (6.63) | 84 (10.73) | |
| No | 854 (89.99) | 155 (93.37) | 699 (89.27) | |
| Surgery primary site, No. (%) | 0.0390* | |||
| Yes | 914 (96.31) | 155 (93.37) | 759 (96.93) | |
| No | 35 (3.69) | 11 (6.63) | 24 (3.07) | |
| Radiation, No. (%) | 0.8690 | |||
| No/unknow | 895 (94.31) | 157 (94.58) | 738 (94.25) | |
| Yes | 54 (5.69) | 9 (5.42) | 45 (5.75) | |
| Chemotherapy, No. (%) | 0.1720 | |||
| No/unknow | 63 (6.64) | 15 (9.04) | 48 (6.13) | |
| Yes | 886 (93.36) | 151 (90.96) | 735 (93.87) | |
| Bone metastasis, No. (%) | 0.1720 | |||
| No | 886 (93.36) | 151 (90.96) | 735 (93.87) | |
| Yes | 63 (6.64) | 15 (9.04) | 48 (6.13) | |
| Brain metastasis, No. (%) | 0.0220* | |||
| No | 881 (92.83) | 161 (96.99) | 720 (91.95) | |
| Yes | 68 (7.17) | 5 (3.01) | 63 (8.05) | |
| Liver metastasis, No. (%) | 0.0830 | |||
| No | 798 (84.09) | 147 (88.55) | 651 (83.14) | |
| Yes | 151 (15.91) | 19 (11.45) | 132 (16.86) | |
| Lung metastasis, No. (%) | <0.001*** | |||
| No | 279 (29.40) | 115 (69.28) | 164 (20.95) | |
| Yes | 670 (70.60) | 51 (30.72) | 619 (79.05) | |
| Lymph node metastasis, No. (%) | 0.0020** | |||
| No | 240 (25.29) | 26 (15.66) | 214 (27.33) | |
| Yes | 709 (74.71) | 140 (84.34) | 569 (72.67) | |
| Metastasis site, No. (%) | 0.0140* | |||
| Lung/lymph node | 712 (75.03) | 129 (77.71) | 583 (74.46) | |
| Bone (± lung/lymph node) | 45 (4.74) | 14 (8.43) | 31 (3.96) | |
| Liver (± lung/lymph node) | 110 (11.59) | 17 (10.24) | 93 (11.88) | |
| Brain (± lung/lymph node) | 40 (4.21) | 4 (2.41) | 36 (4.60) | |
| Multiple nonlung/lymph node sites | 42 (4.43) | 2 (1.20) | 40 (5.11) | |
| Insurance status, No. (%) | 0.5490 | |||
| Uninsured | 104 (10.96) | 16 (9.64) | 88 (11.24) | |
| Insured | 845 (89.04) | 150 (90.36) | 695 (88.76) | |
| Marital status, No. (%) | <0.001*** | |||
| Married | 246 (25.92) | 59 (35.54) | 187 (23.88) | |
| Never married | 636 (67.02) | 89 (53.61) | 547 (69.86) | |
| Othera | 67 (7.06) | 18 (10.84) | 49 (6.26) |
*P<0.05, **P<0.01, ***P<0.001. Othera includes divorced, separated, widowed and unmarried or domestic partner. IQR, interquartile range; NSGCT, nonseminomatous germ cell tumor; Surgery Reg/Dis, surgical removal of distant lymph node(s) or other tissue(s) or organ(s) beyond the primary site.
The median age was 30 years (range, 18–92 years), The majority of the patients (783, 82.51%) had NSGCTs, and 864 (91.04%) were White and 636 (67.02%) had never married. The median follow-up time was 32 months (range, 0–83 months), and 224 (23.60%) patients died before the last follow-up, 193 (20.34%) of whom died due to mTGCT.
Distribution of distant metastatic sites
The distribution of distant metastatic sites is summarized in Figure 2. The distant lymph nodes (709, 74.71%) were the most common location for metastasis, followed by the lungs (670, 70.60%), liver (151, 15.91%) brain (68, 7.17%) and bone (63, 6.64%). Most patients (406, 42.78%) had two sites of distant metastases, followed by a single site (405, 42.68%), three sites (111, 11.60%), four sites (24, 2.53%), and five sites (3, 0.32%). Compared with NSGCTs, patients with SGCTs had a higher proportion of liver (± lung/lymph node), brain (± lung/lymph node), and multiple non-lung/lymph node metastases (P=0.014).

Treatment
In total, 914 patients (96.31%) underwent surgery at the primary site, and 95 (10.01%) had underwent surgery for distant lymph node(s) or other tissue(s) or organ(s) beyond the primary site. Most patients (886, 93.36%) received chemotherapy; the remaining 63 (6.64%) patients either did not receive chemotherapy or their chemotherapy status was unknown. Of the patients who underwent surgery, most (856, 93.65%) received chemotherapy, while 58 patients (6.35%) neither underwent surgery nor received chemotherapy. A small proportion of patients (54, 5.69%) received radiation treatment. The proportion of patients with NSGCTs who underwent surgery at the primary site was higher than that of patients with SGCTs (96.93% vs. 93.37%, P=0.039). No significant differences were found in chemotherapy, radiation, or surgery beyond the primary site between patients with NSGCTs and SGCTs.
The impact of site-specific distant metastases on overall survival
CSS was compared based on different metastatic sites (Figure 3). Kaplan–Meier analyses revealed that among patients with mTGCT, a greater number of metastatic sites (Figure 3B); non-lung/lymph node metastases (Figure 3C); and lung (with vs. without: P=0.0008, Figure 3E), liver (with vs. without: P<0.0001, Figure 3F), bone (with vs. without: P<0.0001, Figure 3G), and brain metastases (with vs. without: P<0.0001, Figure 3H) were associated with significantly poorer survival. For patients with single-site metastases, Kaplan-Meier analyses showed that patients with only distant lymph node metastases had a relatively better CSS rate than that in patients with lung-only, liver-only, and bone-only metastases (P=0.035 for CSS; Figure 3A). Patients with distant lymph node metastases had similar survival outcomes to those in patients without distant lymph node metastases (Figure 3D).
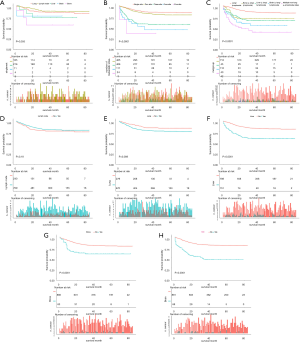
In the entire mTGCT cohort (n=949), based on univariate Cox analysis, age, surgery of primary site, chemotherapy, radiation, site of distant metastases were statistically significant factors of prognosis (Table 2).
Table 2
| Variable | Level | HR | 95% CI | P value |
|---|---|---|---|---|
| Year of diagnosis | ||||
| 2010 | 156 (16.44%) | Reference | ||
| 2011 | 159 (16.75%) | 1.12 | 0.70–1.80 | 0.6252 |
| 2012 | 148 (15.60%) | 1.18 | 0.73–1.91 | 0.4937 |
| 2013 | 189 (19.92%) | 1.18 | 0.75–1.86 | 0.4834 |
| 2014 | 149 (15.70%) | 1.06 | 0.64–1.75 | 0.8322 |
| 2015 | 148 (15.60%) | 0.85 | 0.48–1.53 | 0.5911 |
| Age at diagnosis (years) | 30 (24–40) | 1.02 | 1.01–1.03 | 0.0006*** |
| Laterality | ||||
| Left | 458 (48.26%) | Reference | ||
| Right | 491 (51.74%) | 1.32 | 0.99–1.76 | 0.0567 |
| Histologic type | ||||
| Seminoma | 166 (17.49%) | Reference | ||
| NSGCT | 783 (82.51%) | 1.30 | 0.87–1.94 | 0.2009 |
| Lymphovascular invasion | ||||
| Absent | 511 (53.85%) | Reference | ||
| Present | 438 (46.15%) | 0.85 | 0.64–1.13 | 0.2541 |
| Surgery reg/dis | ||||
| Yes | 95 (10.01%) | Reference | ||
| No | 854 (89.99%) | 1.61 | 0.92–2.83 | 0.0974 |
| Surgery primary site | ||||
| Yes | 914 (96.31%) | Reference | ||
| No | 35 (3.69%) | 3.77 | 2.29–6.21 | <0.0001*** |
| Radiation | ||||
| No/unknow | 895 (94.31%) | Reference | ||
| Yes | 54 (5.69%) | 4.03 | 2.74–5.93 | <0.0001*** |
| Chemotherapy | ||||
| No/unknow | 63 (6.64%) | Reference | ||
| Yes | 886 (93.36%) | 0.32 | 0.21–0.48 | <0.0001*** |
| Bone metastasis | ||||
| No | 886 (93.36%) | Reference | ||
| Yes | 63 (6.64%) | 2.40 | 1.57–3.68 | <0.0001*** |
| Brain metastasis | ||||
| No | 881 (92.83%) | Reference | ||
| Yes | 68 (7.17%) | 3.70 | 2.54–5.38 | <0.0001*** |
| Liver metastasis | ||||
| No | 798 (84.09%) | Reference | ||
| Yes | 151 (15.91%) | 2.96 | 2.17–4.02 | <0.0001*** |
| Lung metastasis | ||||
| No | 279 (29.40%) | Reference | ||
| Yes | 670 (70.60%) | 1.57 | 1.12–2.21 | 0.0089** |
| Lymph node metastasis | ||||
| No | 240 (25.29%) | Reference | ||
| Yes | 709 (74.71%) | 0.81 | 0.59–1.11 | 0.1853 |
| Metastasis site | ||||
| Lung/lymph node | 712 (75.03%) | Reference | ||
| Bone (± lung/lymph node) | 45 (4.74%) | 2.36 | 1.33–4.21 | 0.0035** |
| Liver (± lung/lymph node) | 110 (11.59%) | 2.75 | 1.88–4.02 | <0.0001*** |
| Brain (± lung/lymph node) | 40 (4.21%) | 3.95 | 2.37–6.61 | <0.0001*** |
| Multiple nonlung/lymph node sites | 42 (4.43%) | 6.27 | 4.01–9.80 | <0.0001*** |
| Insurance status | ||||
| Uninsured | 104 (10.96%) | Reference | ||
| Insured | 845 (89.04%) | 0.69 | 0.46–1.04 | 0.0790 |
| Marital status | ||||
| Married | 246 (25.92%) | Reference | ||
| Never married | 636 (67.02%) | 1.24 | 0.88–1.75 | 0.2157 |
| Othera | 67 (7.06%) | 1.25 | 0.69–2.29 | 0.4641 |
*P<0.05, **P<0.01, ***P<0.001. Othera includes divorced, separated, widowed and unmarried or domestic partner. CI, confidence interval; HR, hazard ratio; NSGCT, nonseminomatous germ cell tumor; Surgery reg/dis, surgical removal of distant lymph node(s) or other tissue(s) or organ(s) beyond the primary site.
Multivariate Cox regression analysis for all patients included in this study revealed that the sites of distant metastases were an independent prognostic factor for CSS (Table 3). Compared to those without the corresponding sites of metastases, patients with lung metastases [with vs. without lung metastases: hazard ratio (HR), 1.60; 95% confidence interval (CI), 1.09–2.35; P=0.0157], bone metastases (with vs. without bone metastases: HR, 2.03; 95% CI, 1.29–3.21; P=0.0023), brain metastases (with vs. without brain metastases: HR, 1.98; 95% CI, 1.28–3.06; P=0.0022), and liver metastases (with vs. without liver metastases: HR, 2.27; 95% CI, 1.63–3.17; P<0.0001) revealed worse CSS, while distant lymph node metastases were not an independent prognostic indicator (HR, 1.15; 95% CI, 0.83–1.61; P=0.4003).
Table 3
| Variable | Level | Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR2 | 95% CI | P value | |||
| Age at diagnosis (years) | 30 (24–40) | 1.03 | 1.01–1.04 | <0.0001*** | 1.03 | 1.01–1.04 | <0.0001*** | |
| Laterality | ||||||||
| Left | 458 (48.26%) | Reference | Reference | |||||
| Right | 491 (51.74%) | 1.40 | 1.04–1.88 | 0.0272* | 1.38 | 1.03–1.86 | 0.0328* | |
| Histologic type | ||||||||
| Seminoma | 166 (17.49%) | Reference | Reference | |||||
| NSGCT | 783 (82.51%) | 1.45 | 0.91–2.32 | 0.1189 | 1.74 | 1.11–2.71 | 0.0147* | |
| Lymphovascular invasion | ||||||||
| Absent | 511 (53.85%) | Reference | Reference | |||||
| Present | 438 (46.15%) | 0.93 | 0.68–1.28 | 0.6684 | 1.00 | 0.74–1.36 | 0.9926 | |
| Surgery reg/dis | ||||||||
| Yes | 95 (10.01%) | Reference | Reference | |||||
| No | 854 (89.99%) | 1.61 | 0.91–2.86 | 0.1048 | 1.61 | 0.91–2.85 | 0.1022 | |
| Surgery primary site | ||||||||
| Yes | 914 (96.31%) | Reference | Reference | |||||
| No | 35 (3.69%) | 2.46 | 1.44–4.20 | 0.0009*** | 2.47 | 1.45–4.22 | 0.0009*** | |
| Radiation | ||||||||
| No/unknow | 895 (94.31%) | Reference | Reference | |||||
| Yes | 54 (5.69%) | 2.64 | 1.68–4.12 | <0.0001*** | 2.39 | 1.52–3.76 | 0.0002*** | |
| Chemotherapy | ||||||||
| No/unknow | 63 (6.64%) | Reference | Reference | |||||
| Yes | 886 (93.36%) | 0.28 | 0.17–0.44 | <0.0001*** | 0.29 | 0.19–0.46 | <0.0001*** | |
| Bone metastasis | – | – | – | |||||
| No | 886 (93.36%) | Reference | – | – | – | |||
| Yes | 63 (6.64%) | 2.03 | 1.29–3.21 | 0.0023** | – | – | – | |
| Brain metastasis | – | – | – | |||||
| No | 881 (92.83%) | Reference | – | – | – | |||
| Yes | 68 (7.17%) | 1.98 | 1.28–3.06 | 0.0022** | – | – | – | |
| Liver metastasis | – | – | – | |||||
| No | 798 (84.09%) | Reference | – | – | – | |||
| Yes | 151 (15.91%) | 2.27 | 1.63–3.17 | <0.0001*** | – | – | – | |
| Lung metastasis | – | – | – | |||||
| No | 279 (29.40%) | Reference | – | – | – | |||
| Yes | 670 (70.60%) | 1.60 | 1.09–2.35 | 0.0157* | – | – | – | |
| Lymph node metastasis | – | – | – | |||||
| No | 240 (25.29%) | Reference | – | – | – | |||
| Yes | 709 (74.71%) | 1.15 | 0.83–1.61 | 0.4003 | – | – | – | |
| Metastasis site | ||||||||
| Lung/lymph node | 712 (75.03%) | – | – | – | Reference | |||
| Bone (± lung/lymph node) | 45 (4.74%) | – | – | – | 2.03 | 1.12–3.68 | 0.0189* | |
| Liver (± lung/lymph node) | 110 (11.59%) | – | – | – | 2.59 | 1.74–3.84 | <0.0001*** | |
| Brain (± lung/lymph node) | 40 (4.21%) | – | – | – | 2.61 | 1.47–4.63 | 0.0011** | |
| Multiple nonlung/lymph node sites | 42 (4.43%) | – | – | – | 4.92 | 2.99–8.09 | <0.0001*** | |
| Insurance status | ||||||||
| Uninsured | 104 (10.96%) | Reference | Reference | |||||
| Insured | 845 (89.04%) | 0.69 | 0.45–1.05 | 0.0862 | 0.78 | 0.50–1.20 | 0.2571 | |
*P<0.05, **P<0.01, ***P<0.001. Multivariable Cox regression hazards models were also adjusted for diagnosis year. CI, confidence interval; HR, hazard ratio; Surgery Reg/Dis, surgical removal of distant lymph node(s) or other tissue(s) or organ(s) beyond the primary site.
When they were grouped according to the primary site of metastasis, patients with bone ± lung/lymph node (HR, 2.03; 95% CI, 1.12–3.68; P=0.0189), liver ± lung/lymph node (HR, 2.59; 95% CI, 1.74–3.84; P<0.0001), brain ± lung/lymph node (HR, 2.61; 95% CI, 1.47–4.63; P=0.0011), and multiple non-lung/lymph node (HR, 4.92; 95% CI, 2.99–8.09; P<0.0001) metastases revealed worse prognosis. The histological type (HR, 1.74; 95% CI, 1.11–2.71; P=0.0147 for patients with NSGCTs) became statistically significant in model 2, which was due mostly to adjustments for different groups of metastatic sites. In addition, multivariate Cox analysis indicated that chemotherapy was associated with better CSS in the entire cohort (Table 3). Moreover, patients who had received radiation therapy exhibited worse CSS compared to patients who did not receive radiation therapy (Table 3), even after adjusting for the year of diagnosis, age, surgery, TNM stage, and chemotherapy values.
Development and validation of a nomogram
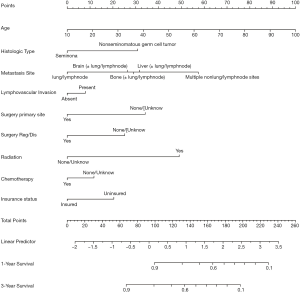
Variables considered to be significantly associated with prognosis were used to develop a nomogram to predict the 1- and 3-year CSS in patients with mTGCTs, as shown in Figure 4.
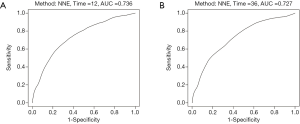
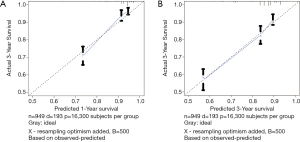
Harrell’s C-index for CSS was 0.739. The AUC values (0.736 and 0.727 for 1- and 3-year CSS, respectively) indicated the good discriminative ability of the nomogram, as shown in Figure 5A,B. Calibration plots showed that the predicted 1- and 3-year survival rates were similar to the actual observations as shown in Figure 6A,B.
Discussion
Previous study indicated that the patient prognosis of several malignancies differs according to the distribution of metastatic involvement (15-20). In this study, using data from a large, nationwide, population-based database, we evaluated the influence of specific metastatic sites on survival in patients with TGCTs, identified independent prognostic factors, and established a nomogram to predict survival. Accurate evaluation of disease prognosis is an important reference value for disease management. The traditional TNM staging system and IGCCCG classification cannot accurately and individually predict patient prognosis because it contains limited prognostic factors (11). Our nomogram can personalize patient outcomes, which can help patients and clinicians choose different management strategies, such as intensified upfront chemotherapy for high-risk patients (21,22). The prognostic factors of mTGCTs are not yet clear. In our study, we found that distant lymph nodes were the most common site of metastases, followed by the lung and liver. Brian and bone metastases were relatively rare. The distribution of distant metastases is consistent with that in previous studies (6,23).
In the survival analysis, we found that patients with distant lymph node metastases revealed the best survival outcomes (although the results were not statistically significant), followed by those with lung, brain, and bone metastases. The prognosis of patients with liver metastasis was the worst. When patients were grouped by primary site of metastasis, non-lung/lymph node metastases were associated with worse prognosis. Patel et al. reported that primary brain metastases confer the worst prognosis (HR =3.24, P<0.01) (6). However, in our study, patients with brain and liver metastases appeared to have similar CSS rates (HR =2.61, P=0.0011 for brain metastases and HR =2.59, P<0.0001 for liver metastases). This result may be explained by the fact that we included patients with only distant lymph node metastases, and we also adjusted for the influence of treatment regimens in multivariate Cox regression analysis. One possible reason for poor prognosis in patients with metastasis is tumor’s resistance to conventional treatment (9,23-25).
Our nomogram used the following prognostic factors, which were shown in previous studies to be associated with survival in patients with mTGCT: age at diagnosis (26); surgical status of primary site and metastatic site (8); chemotherapy (27); radiotherapy; insurance status (28) and whether has liver (23,24), lung (28), bone (23), and brain (23) metastasis, lymphovascular invasion (29), and histology (29). As far as we know, this is the first study in which a nomogram to predict CSS for patients with mTGCTs. The AUC values for 1- and 3-year CSS were 0.736 and 0.727, respectively, indicating the good discriminative ability of the nomogram.
We acknowledge that there are some limitations to our research. First, our study was retrospective in nature, with inevitable selection bias. Second, the SEER database only captured lung, liver, bone, brain, and lymph node distant metastatic sites. Therefore, we were unable to compare survival rates associated with other metastatic sites, although for patients with TGCTs, these are the most common metastatic sites. Third, there was a lack of information about treatment strategies, family history, serum tumor markers and the size of metastatic lesions, which may cause bias. Fourth, a previous study indicated that there are some errors regarding TNM staging in SEER database (30). However, these errors were largely due to the S and N categories, and we had already avoided those factors in this study. Finally, external validation is essential to prove the accuracy and clinical utility of our models. However, this was a real-world study based on a large sample size, and these limitations do not weaken our conclusions.
Conclusions
In summary, the site of distant metastasis is an independent prognostic factor for cancer specific survival. We developed a nomogram to predict the 1- and 3-year CSS of patients with mTGCTs, which can help patients and clinicians accurately predict mortality risk and recommend a personalized treatment modality.
Acknowledgments
The authors are grateful to all the staff at the National Cancer Institute (USA) for their contribution to the SEER program.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.03.59). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sarıcı H, Telli O, Eroglu M. Bilateral testicular germ cell tumors. Turk J Urol 2013;39:249-52. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Huyghe E, Matsuda T, Thonneau P. Increasing incidence of testicular cancer worldwide: a review. J Urol 2003;170:5-11. [Crossref] [PubMed]
- Gurney JK, Florio AA, Znaor A, et al. International Trends in the Incidence of Testicular Cancer: Lessons from 35 Years and 41 Countries. Eur Urol 2019;76:615-23. [Crossref] [PubMed]
- Woldu SL, Matulay JT, Clinton TN, et al. Impact of hospital case volume on testicular cancer outcomes and practice patterns. Urol Oncol 2018;36:14.e7-5. [Crossref] [PubMed]
- Patel HD, Singla N, Ghandour RA, et al. Site of extranodal metastasis impacts survival in patients with testicular germ cell tumors. Cancer 2019;125:3947-52. [Crossref] [PubMed]
- Jamal-Hanjani M, Karpathakis A, Kwan A, et al. Bone metastases in germ cell tumours: lessons learnt from a large retrospective study. BJU Int 2013;112:176-81. [Crossref] [PubMed]
- Feldman DR, Lorch A, Kramar A, et al. Brain Metastases in Patients With Germ Cell Tumors: Prognostic Factors and Treatment Options--An Analysis From the Global Germ Cell Cancer Group. J Clin Oncol 2016;34:345-51. [Crossref] [PubMed]
- Copson E, McKendrick J, Hennessey N, et al. Liver metastases in germ cell cancer: defining a role for surgery after chemotherapy. BJU Int 2004;94:552-8. [Crossref] [PubMed]
- Collins GS, Reitsma JB, Altman DG, et al. Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD): The TRIPOD Statement. Br J Cancer 2015;112:251-9. [Crossref] [PubMed]
- Kim SY, Yoon MJ, Park YI, et al. Nomograms predicting survival of patients with unresectable or metastatic gastric cancer who receive combination cytotoxic chemotherapy as first-line treatment. Gastric Cancer 2018;21:453-63. [Crossref] [PubMed]
- Zi H, Gao L, Yu Z, et al. Nomograms for predicting long-term overall survival and cancer-specific survival in patients with primary urethral carcinoma: a population-based study. Int Urol Nephrol 2020;52:287-300. [Crossref] [PubMed]
- Kutikov A, Egleston BL, Wong YN, et al. Evaluating overall survival and competing risks of death in patients with localized renal cell carcinoma using a comprehensive nomogram. J Clin Oncol 2010;28:311-7. [Crossref] [PubMed]
- Tang F, He Z, Lu Z, et al. Application of nomograms in the prediction of overall survival and cancer-specific survival in patients with T1 high-grade bladder cancer. Exp Ther Med 2019;18:3405-14. [PubMed]
- Deng K, Yang C, Tan Q, et al. Sites of distant metastases and overall survival in ovarian cancer: A study of 1481 patients. Gynecol Oncol 2018;150:460-5. [Crossref] [PubMed]
- Dong F, Shen Y, Gao F, et al. Prognostic value of site-specific metastases and therapeutic roles of surgery for patients with metastatic bladder cancer: a population-based study. Cancer Manag Res 2017;9:611-26. [Crossref] [PubMed]
- Chandrasekar T, Klaassen Z, Goldberg H, et al. Metastatic renal cell carcinoma: Patterns and predictors of metastases-A contemporary population-based series. Urol Oncol 2017;35:661.e7-14. [Crossref] [PubMed]
- Budnik J, Suri J, Bates JE, et al. Prognostic Significance of Sites of Visceral Metastatic Disease in Prostate Cancer: A Population-based Study of 12,180 Patients. Clin Genitourin Cancer 2019;17:260-7. [Crossref] [PubMed]
- Zhao Z, Wu W, Duan X, et al. The value of cytoreductive nephrectomy on the survival of metastatic renal carcinoma patients based on the number of site-specific metastases. PLoS One 2019;14:e0215861. [Crossref] [PubMed]
- Zhang C, Liu L, Tao F, et al. Bone Metastases Pattern in Newly Diagnosed Metastatic Bladder Cancer: A Population-Based Study. J Cancer 2018;9:4706-11. [Crossref] [PubMed]
- Daugaard G, Skoneczna I, Aass N, et al. A randomized phase III study comparing standard dose BEP with sequential high-dose cisplatin, etoposide, and ifosfamide (VIP) plus stem-cell support in males with poor-prognosis germ-cell cancer. An intergroup study of EORTC, GTCSG, and Grupo Germinal (EORTC 30974). Ann Oncol 2011;22:1054-61. [Crossref] [PubMed]
- Huddart RA, Gabe R, Cafferty FH, et al. A randomised phase 2 trial of intensive induction chemotherapy (CBOP/BEP) and standard BEP in poor-prognosis germ cell tumours (MRC TE23, CRUK 05/014, ISRCTN 53643604). Eur Urol 2015;67:534-43. [Crossref] [PubMed]
- International Prognostic Factors Study Group. Prognostic Factors in Patients With Metastatic Germ Cell Tumors Who Experienced Treatment Failure With Cisplatin-Based First-Line Chemotherapy. J Clin Oncol 2010;28:4906-11. [Crossref] [PubMed]
- Pietzak EJ, Assel M, Becerra MF, et al. Histologic and Oncologic Outcomes Following Liver Mass Resection With Retroperitoneal Lymph Node Dissection in Patients With Nonseminomatous Germ Cell Tumor. Urology 2018;118:114-8. [Crossref] [PubMed]
- Oechsle K, Kollmannsberger C, Honecker F, et al. Cerebral metastases in non-seminomatous germ cell tumour patients undergoing primary high-dose chemotherapy. Eur J Cancer 2008;44:1663-9. [Crossref] [PubMed]
- Terbuch A, Posch F, Bauernhofer T, et al. Age as a Predictor of Treatment Outcome in Metastatic Testicular Germ Cell Tumors. Anticancer Res 2019;39:5589-96. [Crossref] [PubMed]
- Albany C, Adra N, Snavely AC, et al. Multidisciplinary clinic approach improves overall survival outcomes of patients with metastatic germ-cell tumors. Ann Oncol 2018;29:341-6. [Crossref] [PubMed]
- Palumbo C, Mistretta FA, Mazzone E, et al. Contemporary Incidence and Mortality Rates in Patients With Testicular Germ Cell Tumors. Clin Genitourin Cancer 2019;17:e1026-35. [Crossref] [PubMed]
- Hanna NH, Einhorn LH. Testicular cancer--discoveries and updates. N Engl J Med 2014;371:2005-16. [Crossref] [PubMed]
- Faber KD, Carlos MC, Cortessis VK, et al. Validation of Surveillance, Epidemiology, and End Results TNM staging for testicular germ cell tumor. Urol Oncol 2014;32:1341-6. [Crossref] [PubMed]