Neurological paraneoplastic syndrome caused by small cell lung cancer: a case report
Introduction
The main treatments for neurological neoplastic syndrome (PNS) which is specifically diagnosed by Hu antibody are anti-tumor therapy and immune therapy. The first-line approach to treatment involves localizing and treating primary tumor, in order to clear associated antigens. However, even resection or chemoradiotherapy of the primary tumor does not alleviate the symptoms associated with PNS. Immunomodulatory therapy may help in treating PNS symptoms involving peripheral nerves at the nerve-muscle interface or joints, but this approach provides little benefit in alleviating symptoms that affect the central nervous system (CNS). Though most patients submit to aggressive treatment for PNS, most are not satisfied with the results (1). Irreversible injury to neurons of the CNS leads to lasting damage. This case represents an optimal approach to treatment of PNS and may serve as a reference for clinicians. We present the following case in accordance with the CARE Guideline.
Case presentation
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent provided is available for review by the Editor-in-Chief of this journal.
A 59-year-old male presented with numbness of the face and limbs, which had previously been misdiagnosed as peripheral neuritis. Numbness persisted despite treatment with methylcobalamin (vitamin B12), which had been prescribed by the previous physician. When the patient was 29 years old, the patient had previously undergone right pulmonary bulla resection and recovered uneventfully. The patient had no other relevant medical history and no family history of tumor. Chest computed tomography (CT) at our institution revealed nodular density in the patient’s left lower lobe.
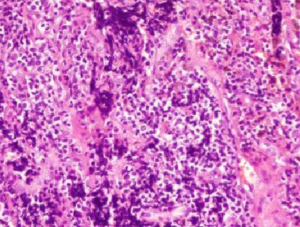
The patient underwent surgical treatment on October 14, 2015. Postoperative pathology identified small-cell lung carcinoma (SCLS) in the left lower lobe (Figure 1). Immunohistochemical staining was positive for Syn, CgA, CD56, TTF-1, CK (AE1/AE3), Hu antibodies (Table 1), and Ki67 (90%). Immunohistochemical staining was negative for LCA, P40, and CK5/6 was negative. Immunohistochemical staining was positive for Syn, CgA, CD56, TTF-1, and CK (AE1/AE3) (Figure 2). This patient was clinically diagnosed with a localized stage of small cell lung cancer and PNS. After surgery, numbness initially decreased but then started to return 3 days postoperatively. The patient received two cycles of chemotherapy (etoposide + platinum) with immune modulation during the period from October 2015 to December 2015. After each treatment, the patient’s numbness was alleviated for 2–3 days but then returned to baseline. The patient received radiation therapy, but this approach was discontinued because of bone marrow suppression. The patient’s numbness persisted, causing him to contemplate suicide. A multidisciplinary team prescribed pregabalin, bulleyaconitine A, amitriptyline, and duloxetine. This pharmacologic regimen alleviated the patient’s symptoms. The dosage of the four drugs were used for half a year according to the instructions, and the clinical symptoms were relieved 3 weeks later. This patient had been taking prabalinda orally for 1 year. No obvious adverse reactions or adverse events were observed. The patient had good compliance and no obvious adverse drug reactions. He is regularly followed-up in our hospital. After reexamination in the local hospital, there was no obvious abnormal disease in the patient. The patient remained alive and well at follow-up in December 2018. So far, patients have undergone chest CT and abdominal color Doppler ultrasound, showing that the tumor is stable, and the symptoms alleviated significantly.
Table 1
| Basic information | Experimental result | Conclusions and tips | |
|---|---|---|---|
| Corresponding antibody | Result | ||
| Sex: male | Amphiphysin | Negative | The serum Hu antibody of the patient was positive (++) |
| Age: 59 years old | CV2.1 | Negative | |
| Admission number | PNMA2(Ma2/Tx) | Negative | |
| Sample type: serum | Ri | Negative | |
| Number | Yo | Negative | |
| Department: neurology | Hu | ++ | |
| Submission date: 2016-3-10 | Control | +++ | |
| Report date: 2016-3-14 | Inspector | Yuehua GU | |
Discussion
The pathogenesis of PNS is a complex remote effect of the tumor. Antigens expressed by tumor cells may trigger immune cross-reactivity with nerve tissue, resulting in nervous system dysfunction. The immune response may also be triggered by the rapid growth and apoptosis of tumor cells, which are engulfed by antigen-presenting cells. Abnormal neural differentiation may result in aberrant expression of nerve antigen, which can also trigger the immune response.
Hu antibody is highly sensitive and specific in the diagnosis of SCLC with PNS. Sentis Madrid et al. report that positive immunostaining for Hu antibody in combination with the classic clinical syndrome (e.g., numbness of the face and limbs, suicidal depression) is sufficient for the diagnosis of SCLC with PNS, as in the case presented here.
To date, no randomized controlled trial has investigated treatment for PNS. Current approaches include: (I) anti-cancer treatments to control disease progression (e.g., surgery, radiotherapy, chemotherapy); (II) immunosuppressive therapy, such as hormones, cyclophosphamide, plasma exchange, humoral immune treatment (2). Most patients are ultimately unsatisfied with the results of immune therapy. The main approach to treatment remains control of the primary tumor.
In this case, a multidisciplinary team prescribed adjuvant therapy (pregabalin, bulleyaconitine A, amitriptyline, and duloxetine) to treat the patient’s primary disease. Numbness in the patient’s face and limbs had decreased by 1 month after treatment. Pregabalin reduces the release of excitatory neurotransmitters such as glutamate, noradrenaline, and substance P, which helps in the treatment of neuropathic pain and allergic symptoms (3). Bulleyaconitine A, a modern plant medicine, has proven analgesic and anti-inflammatory effects (4), which are mediated by effects on the transmission of sodium-ion current (5). Similar mechanisms underlie the effects of duloxetine and amitriptyline. These drugs enhance the activity of norepinephrine and serotonin in nerve conduction, which is useful for the regulation of emotion and pain sensitivity and can improve pain tolerance (6). These drugs are also effective in treating peripheral neuropathy induced by chemotherapy (7).
The treatment regimen outlined above alleviated the patient’s PNS symptoms. Treatment may have decreased secretion of abnormal hormones from tumor cells, weakening the immune system response. Treatment thus prevented permanent neurological damage, improving the patient’s quality of life and prolonging survival.
This study found that prognosis and survival rate were superior among patients expressing HuAb compared to those who did not. This phenomenon may be related to the immune response mediated by cytotoxic T cells (8). Thus HuAb positivity represents an important marker in the early diagnosis of SCLC (9). However, positivity for HuAb may falsely flag patients who do not have SCLC (10).
Conclusions
The incidence of PNS in patients with tumors is low. Conventional treatment achieves unsatisfactory results, leading to poor prognosis. In this case report, treatment with pregabalin and antidepressant agents improved the patient’s numbness. This case may inform future treatment of patients with PNS. Because the patient received this treatment alone, the conclusion has some limitations, which need to be verified by a large number of patients.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.03.54). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Consent was obtained from relatives of the patient for publication of this report and any accompanying images. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Grisold W, Giometto B, Vitaliani R, et al. Current approaches to the treatment of paraneoplastic encephalitis. Ther Adv Neurol Disord 2011;4:237-48. [Crossref] [PubMed]
- Giometto B, Vitaliani R, Lindeck-Pozza E, et al. Treatment for paraneoplastic neuropathies. Cochrane Database Syst Rev 2012;12:CD007625. [PubMed]
- De Santis S, Borghesi C, Ricciardi S, et al. Analgesic effectiveness and tolerability of oral oxycodone/naloxone and pregabalin in patients with lung cancer and neuropathic pain: an observational analysis. Onco Targets Ther 2016;9:4043-52. [Crossref] [PubMed]
- Firestein GS. Invasive fibroblast-like synoviocytes in rheumatoid arthritis. Passive responders or transformed aggressors? Arthritis Rheum 1996;39:1781-90. [Crossref] [PubMed]
- Wang CF, Gerner P, Wang SY, et al. Bulleyaconitine A isolated from aconitum plant displays long-acting local anesthetic properties in vitro and in vivo. Anesthesiology 2007;107:82-90. [Crossref] [PubMed]
- Gupta S, Nihalani N, Masand P. Duloxetine: review of its pharmacology, and therapeutic use in depression and other psychiatric disorders. Ann Clin Psychiatry 2007;19:125-32. [Crossref] [PubMed]
- Saif MW, Syrigos K, Kaley K, et al. Role of pregabalin in treatment of oxaliplatin-induced sensory neuropathy. Anticancer Res 2010;30:2927-33. [PubMed]
- Gill S, Murray N, Dalmau J, et al. Paraneoplastic sensory neuronopathy and spontaneous regression of small cell lung cancer. Can J Neurol Sci 2003;30:269-71. [Crossref] [PubMed]
- Stefens-Stawna P, Piorunek T, Gabryel-Batura H, et al. Neurological paraneoplastic syndromes in lung cancer patients. Adv Exp Med Biol 2013;756:333-9. [Crossref] [PubMed]
- Titulaer MJ, Lang B, Verschuuren JJ. Lambert-Eaton myasthenic syndrome: from clinical characteristics to therapeutic strategies. Lancet Neurol 2011;10:1098-107. [Crossref] [PubMed]