
LAMP1 is more sensitive than LAMP2 in predicting prognosis of esophageal squamous cell carcinoma
Introduction
Esophageal squamous cell carcinoma (ESCC), the fourth most common cause of cancer-related deaths, is one of the most common cancers, especially in China (1,2). The 5-year survival rate of ESCC remains very low (3), which was closely related to tumor invasion and metastasis (4). At present, only less than 30% of ESCC patients can achieve early diagnosis and treatment. To improve the clinical outcome of ESCC, novel molecular biomarkers used for early diagnosis and predicting prognosis have been widely investigated (5,6).
Lysosomes contain more than 60 kinds of acid hydrolases and various lysosomal specific membrane proteins, whose main function is to receive and degrade macromolecules, including cellular components derived from autophagy, such as damaged organelles, aging and misfolded macromolecules (7). Lysosomal associated membrane proteins (LAMPs), including LAMP1 and LAMP2, are the major protein components of lysosomes (8-10), which also play an important role in tumor evolution (11,12). It has been confirmed that the LAMP1 gene showed high expression in astrocytoma (13). Furthermore, LAMP1 has also been found expressing on the cell surface of highly metastatic tumor cells, suggesting a role for LAMP1 in tumor cells migration (14-16).
In our previous studies, we detected the expression of LAMP1 and LAMP2 in the surgically resected tissues of nearly 600 patients with ESCC, the results of which have also confirmed that LAMP1 and LAMP2 play an important role in the evolution and prognosis of ESCC (17-19). To further explore their roles in ESCC, we compared the roles of LAMP1 and LAMP2 in predicting the prognosis of ESCC in the present study.
Methods
Patients’ information
Five hundred seventy nine ESCC patients with complete IHC results and prognosis data were enrolled in this study. The clinical information, address and telephone number of patients were collected from the medical records. The prognosis data were provided by the Follow-up Center of Affiliated Hospital of Jining Medical University. This study was reviewed and approved by the Medical Ethics Committee of Affiliated Hospital of Jining Medical University (No. 2017-FY-007). Informed consent was obtained from all patients.
Immunohistochemistry staining (IHC)
In our previous studies, IHC of LAMP1 and LAMP2 was performed simultaneously under the same conditions. The experimental protocols and criteria for determining results have been reported. And the results of IHC were divided into four grades: “−”, “+”, “++”, and “+++”, which has also been reported in our previous studies (17,18). It should be noted that all results in this study are the first to be reported.
Statistical analysis
Kaplan-Meier survival curves and receiver operating characteristic (ROC) curves were performed using SPSS software 22.0. P<0.05 was considered as statistically significant.
Results
Clinical data
After summarizing our previous research data, 579 patients with complete IHC results and prognosis data were enrolled in this study. The patients’ information and IHC results are listed in Table 1 and Figure 1. The patient’s survival rates were calculated simply according to the patient’s survival, based on the deletion of some recent cases. The 3-year survival rate was 51.91% (231/445), and the 5-year survival rate was 34.58% (83/240).
Table 1
| Item | LW | LE | LH |
|---|---|---|---|
| Gender | |||
| Female | 30 | 55 | 45 |
| Male | 101 | 196 | 152 |
| Age | |||
| <60 | 53 | 109 | 81 |
| ≥60 | 78 | 142 | 116 |
| TNM | |||
| I | 1 | 6 | 7 |
| II | 85 | 145 | 122 |
| III | 45 | 100 | 68 |
| Metastasis* | |||
| No | 46 | 87 | 50 |
| Yes | 26 | 46 | 41 |
| Differentiation** | |||
| Well | 1 | 1 | 7 |
| Well-moderate | 28 | 33 | 25 |
| Moderate | 29 | 65 | 83 |
| Moderate-poor | 58 | 105 | 68 |
| Poor | 15 | 47 | 14 |
*, some cases were not sure whether metastasis occurred; **, χ2=40.074, P<0.001. LW: LAMP1 < LAMP2; LE: LAMP1 = LAMP2; LH: LAMP1 > LAMP2. ESCC, esophageal squamous cell carcinoma.
Kaplan-Meier survival curves
Firstly, the positive degrees were redefined. “−” and “+” were defined as the low expression (−). “++” and “+++” were defined as high expression (+). Then, we divided all cases into four groups according to the results of IHC: LAMP1(−)LAMP2(−), LAMP1(−)LAMP2(+), LAMP1(+)LAMP2(−), LAMP1(+)LAMP2(+). Kaplan-Meier survival curves indicated that there was no statistical difference between the four groups. However, it is interesting that the survival curve of patients with similar expression of LAMP1 and LAMP2 tended to be consistent, and that the difference between the other two groups was very obvious (Figure 2A).
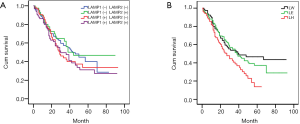
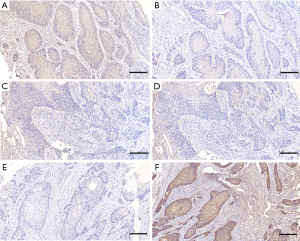
Based on the above results, we adjusted the grouping method. We divided all cases into three groups: LAMP1 high expression group (LH, LAMP1>LAMP2, Figure 1A,B), expression equivalent group (LE, LAMP1 = LAMP2, Figure 1C,D), and LAMP1 low expression group (LW, LAMP1 < LAMP2, Figure 1E,F). Then, we used Kaplan-Meier survival curves again to analyze the prognosis difference of the three groups. The Kaplan-Meier survival curves indicated that the prognosis of group LH was the worst, followed by group LE and group LW. In other words, compared with LAMP2, the higher the expression level of LAMP1, the worse the prognosis of patients (Figure 2B, P<0.05).
ROC curves
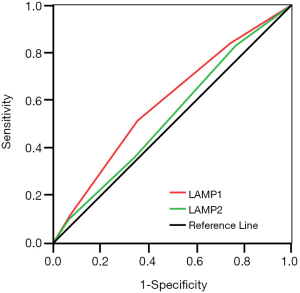
Additionally, we used the ROC curve to compare the sensitivity and specificity of LAMP1 and LAMP2 in predicting prognosis of ESCC patients. The area under the curve (AUC) of LAMP1 was significantly higher than that of LAMP2 (Figure 3, AUCLAMP1 =0.593, AUCLAMP2 =0.534, P<0.05), which indicated that LAMP1 is a more sensitive marker than LAMP2 in the prognosis of ESCC.
Discussion
LAMP1 and LAMP2, markers of the lysosomes, are localized at the lysosomal membrane under physiological conditions, playing an important role in the lysosomal physiological process (20,21). LAMP1 and LAMP2 share most common functions, but they also play different roles in some other aspects, such as lysosome transport, exocytosis, chaperone-mediated autophagy, autophagy-lysosome fusion and cholesterol transport (22). In terms of malignant tumors, LAMP1 has been identified expressing higher in many cancers, especially in metastatic cancer cells, playing an important role in tumor invasion and metastasis, but LAMP2 cannot (14,16,23).
In the present study, the Kaplan-Meier survival curves showed that the sensitivity of LAMP1 was significantly higher than that of LAMP2 in the prognosis of ESCC. Compared to LAMP2, the higher the expression of LAMP1, the worse the prognosis of ESCC patients. To further confirm this result, we performed a ROC curve analysis of the sensitivity. The AUC of LAMP1 was significantly higher than that of the LAMP2, indicating that the LAMP1 is more sensitive than LAMP2 in predicting prognosis of ESCC. We will continue to study its molecular mechanism in the following studies.
Additionally, it needs to be pointed out that there are still some other defects in the present study. Firstly, the small sample size of some subgroups (Table 1) makes it impossible for us to conduct more in-depth analysis between subgroups; meanwhile, the defects of data may affect, to a certain extent, the accuracy of our statistical analysis. Secondly, this study only focused on the statistical analysis of clinical data and IHC results, but failed to explore the molecular mechanisms of LAMP1 and LAMP2 associated with the prognosis of ESCC. So, our results still need to be further validated.
Conclusions
LAMP1 is more sensitive than LAMP2 in predicting the prognosis of ESCC, which indicated that LAMP1 may play a more important role in the evolution and metastasis of ESCC.
Acknowledgments
Funding: This study was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.03.27). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was reviewed and approved by the Medical Ethics Committee of Affiliated Hospital of Jining Medical University (No. 2017-FY-007). Informed consent was obtained from all patients. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Bao Y, Liu S, Zhou Q, et al. Three-dimensional conformal radiotherapy with concurrent chemotherapy for postoperative recurrence of esophageal squamous cell carcinoma: clinical efficacy and failure pattern. Radiat Oncol 2013;8:241. [Crossref] [PubMed]
- Rice TW, Rusch VW, Apperson-Hansen C, et al. Worldwide esophageal cancer collaboration. Dis Esophagus 2009;22:1-8. [Crossref] [PubMed]
- Huang Q, Luo K, Chen C, et al. Identification and Validation of Lymphovascular Invasion as a Prognostic and Staging Factor in Node-Negative Esophageal Squamous Cell Carcinoma. J Thorac Oncol 2016;11:583-92. [Crossref] [PubMed]
- Wu C, Wang Z, Song X, et al. Joint analysis of three genome-wide association studies of esophageal squamous cell carcinoma in Chinese populations. Nat Genet 2014;46:1001-6. [Crossref] [PubMed]
- Hale AN, Ledbetter DJ, Gawriluk TR, et al. Autophagy: regulation and role in development. Autophagy 2013;9:951-72. [Crossref] [PubMed]
- Saftig P, Klumperman J. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat Rev Mol Cell Biol 2009;10:623-35. [Crossref] [PubMed]
- Schröder BA, Wrocklage C, Hasilik A, et al. The proteome of lysosomes. Proteomics 2010;10:4053-76. [Crossref] [PubMed]
- Hook G, Jacobsen JS, Grabstein K, et al. Cathepsin B is a New Drug Target for Traumatic Brain Injury Therapeutics: Evidence for E64d as a Promising Lead Drug Candidate. Front Neurol 2015;6:178. [Crossref] [PubMed]
- Hämälistö S, Jaattela M. Lysosomes in cancer-living on the edge (of the cell). Curr Opin Cell Biol 2016;39:69-76. [Crossref] [PubMed]
- Liao X, Chen Y, Liu D, et al. High Expression of LAMP3 Is a Novel Biomarker of Poor Prognosis in Patients with Esophageal Squamous Cell Carcinoma. Int J Mol Sci 2015;16:17655-67. [Crossref] [PubMed]
- Jensen SS, Aaberg-Jessen C, Christensen KG, et al. Expression of the lysosomal-associated membrane protein-1 (LAMP-1) in astrocytomas. Int J Clin Exp Pathol 2013;6:1294-305. [PubMed]
- Agarwal AK, Srinivasan N, Godbole R, et al. Role of tumor cell surface lysosome-associated membrane protein-1 (LAMP1) and its associated carbohydrates in lung metastasis. J Cancer Res Clin Oncol 2015;141:1563-74. [Crossref] [PubMed]
- Saitoh O, Wang WC, Lotan R, et al. Differential glycosylation and cell surface expression of lysosomal membrane glycoproteins in sublines of a human colon cancer exhibiting distinct metastatic potentials. J Biol Chem 1992;267:5700-11. [PubMed]
- Agarwal AK, Gude RP, Kalraiya RD. Regulation of melanoma metastasis to lungs by cell surface Lysosome Associated Membrane Protein-1 (LAMP1) via galectin-3. Biochem Biophys Res Commun 2014;449:332-7. [Crossref] [PubMed]
- Huang J, Li L, Liu J, et al. Altered expression of lysosomal associated membrane protein 1 in esophageal squamous cell carcinoma. Pathol Res Pract 2017;213:938-942. [Crossref] [PubMed]
- Li L, Wang W, Zhang R, et al. High expression of LAMP2 predicts poor prognosis in patients with esophageal squamous cell carcinoma. Cancer Biomark 2017;19:305-311. [Crossref] [PubMed]
- Wang W, Zhang Y, Xu Y, et al. Prognostic value of LAMP1 in surgically resected esophageal squamous cell carcinoma. Transl Cancer Res 2018;7:347-52. [Crossref]
- Hasilik A, Wrocklage C, Schroder B. Intracellular trafficking of lysosomal proteins and lysosomes. Int J Clin Pharmacol Ther 2009;47:S18-33. [PubMed]
- Xu H, Ren D. Lysosomal physiology. Annu Rev Physiol 2015;77:57-80. [Crossref] [PubMed]
- Schneede A, Schmidt CK, Holtta-Vuori M, et al. Role for LAMP-2 in endosomal cholesterol transport. J Cell Mol Med 2011;15:280-95. [Crossref] [PubMed]
- Krishnan V, Bane SM, Kawle PD, et al. Altered melanoma cell surface glycosylation mediates organ specific adhesion and metastasis via lectin receptors on the lung vascular endothelium. Clin Exp Metastasis 2005;22:11-24. [Crossref] [PubMed]


