Impact of oral mucositis on quality of life in patients undergoing oncological treatment: a systematic review
Introduction
Antineoplastic therapy has been witnessing continuous improvement in terms of overall survival rates and progression free survival; nevertheless, it is still accompanied by a cluster of side effects affecting the quality of life (QoL) (1,2). While this compromise might be medically reasonable (tumor suppression > QoL), many patients especially the elderly and patients with advanced malignancies consider their QoL as the most important factor in survival (3,4). The quality of life (QoL) in oncology is a multidimensional paradigm and has generated considerable interest over the past few years. QoL usually assesses four dimensions: physical well-being which refers to apparent bodily function; functional well-being which is the capacity to perform normal daily activities; emotional well-being involving positive and negative aspects and social well-being which is the ability to maintain social relationships and social life (3,4). It outlines the perception of the survivor to the impact of cancer and its management as well as its effect on different facets of life, including physical and psychological well-being (3,4).
Antineoplastic therapy induced oral mucositis (OM) is known to have a significant impact on the QoL (5). It is characterized by the presence of erythema and edema of the oral and oropharyngeal mucosa culminating in ulcerative erosive lesions 3–5 days or 7–10 days from the initiation of chemotherapy (CT) or radiotherapy (RT) respectively (6,7). Targeted cancer therapies have shown to exert similar oral manifestations with different clinical characteristics (8). Incidence of OM varies according to the degree of toxicity of the antineoplastic regimen used, patient’s age, oral hygiene protocol followed and several other systemic factors (9). OM has been reported to affect 20–40% of patients who receive conventional chemotherapy and 75–85% of patients who undergo bone marrow transplantation (6-10). The incidence can be as high as 100% in patients who receive radiotherapy for head and neck malignancies (10-12) due to the proximity in location. In addition to the decline in oral health, the occurrence of OM is accompanied by difficulty in chewing, swallowing, eating and drinking, as a consequence of pain and inflammation of the oral mucosa and the esophagus. If untreated, the debilitating symptoms can lead to reduced appetite leading to alterations in nutrition, which, if severe enough, can lead to interruptions in treatment (13). Moreover, OM can increase the risk of systemic sepsis and in severe cases, it may necessitate antineoplastic treatment dose reduction or even treatment cessation, affecting the survival chances of the cancer patient (14-16). OM management relies heavily on opioid analgesics since most of the interventions have not been able to provide complete alleviation (17,18).
Measurements for the investigation of OM is a complex scenario due to its heterogeneity in symptoms that varies with the type of CT/RT and type of cancer. Several studies have assessed the QoL outcomes in OM in oncological patients; however, it remains a challenge for the oncologist to determine which studies have reported these assessments with objectivity, to impact the decision making in comprehensive patient care. We aim to systematically review the literature to provide a qualitative assessment of the current evidence on the impact of OM on QoL in patients undergoing oncologic treatment. This approach can help to integrate all the relevant evidence on OM and QoL and identify the research priorities to fill the gaps.
Methods
A systematic search for studies was performed in MEDLINE and Embase databases from inception to December 2018 using the MeSH terms for the keywords “Antineoplastic”, “Stomatitis”, and “Quality of life”. Studies that met the following eligibility criteria were included: study participants were adult patients receiving cancer treatment; study design was a prospective study evaluating the impact of OM on the QoL; a validated instrument was used to measure the QoL. The exclusion criteria excluded; studies which evaluated the QoL in non-oncological treatment; studies which compared treatments or interventions for OM; and studies which did not use a validated measurement tool for assessing QoL. The search strategy was limited to English language comprising of human studies only. Studies were initially assessed and included/excluded based on the title and abstract followed by a full text review. Data extraction was performed independently by two reviewers (AHM Al-Rudayni, D Gopinath). Information of included trials were extracted into a standardized data collection form, comprising author/year, study design, study characteristics, mucositis assessment, QoL assessment and results. Any disagreements were resolved by consensus among the reviewers.
Results
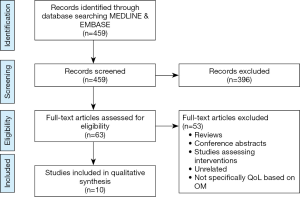
A total of 459 articles were selected after removal of duplicates. Sixty-three articles were selected for full review after screening of title and abstracts. Following the full text review, only ten articles qualified for the systematic review based on the selection criteria (Figure 1).
Participants
The selected articles were all prospective studies, published between 2001–2018 (19-28) (Table 1). All the participants in the selected studies were adults between 18 and 80 years. The number of participants in each study ranged from 20–322. All the participants were receiving either chemotherapy, radiotherapy or both.
Table 1
| No | Author, year (ref.) | Region | Cancer type | Study subjects details | Study design | Mucositis assessment | QoL assessment | Results |
|---|---|---|---|---|---|---|---|---|
| 1 | Dodd et al., 2001 (19) | US | Solid | 77 patients: 28 (OM), 49 (no OM). | Prospective, longitudinal, observational | Oral Assessment Guide (OAG) | MQoLS-CA, POMS | Comparison start of chemotherapy/start of mucositis or end of study: |
| Mean MQOLS-CA score: OM 69.92/62.24; no OM 75.33/69.44 | ||||||||
| Depression: OM 3.43/7.57, no OM 3.69/4.31, P<0.001 | ||||||||
| Anger: OM 2.50/5.07, no OM 3.21/3.53, P<0.01 | ||||||||
| Anxiety: OM 5.46/6.79, no OM 4.89/4.58, NS | ||||||||
| Confusion: OM 4.22/4.57, no OM 2.75/3.15, NS | ||||||||
| Vigor: OM 7.89/5.36, no OM 9.76/8.29, NS | ||||||||
| Fatigue: OM 7.54/11.54, no OM 5.19/7.69, NS | ||||||||
| Total mood disturbance: OM 15.26/30.18, no OM 9.98/14.97, P=0.03 | ||||||||
| 2 | Epstein et al., 2007 (20) | Canada | HNC | HNC with RT; 20 patients | Prospective, longitudinal, observational | Patient reported symptoms (MTS) | FACT-G OMWQ‐HN | Mean scores before cancer therapy/1 month/6 months after treatment: |
| Overall QoL: 5.65/3.40/4.45, P<0.0005 | ||||||||
| Overall physical: 5.5/3.4/4.3, P<0.0005 | ||||||||
| Physical: 96.0/51.0/78.0, P<0.0005 | ||||||||
| Social: 86.7/53.3/74.2, P<0.0005 | ||||||||
| Role: 95.0/45.0/80.0, P<0.0005 | ||||||||
| Global: 76.3/40.0/56.7, P<0.0005 | ||||||||
| Cognitive: 85.0/67.5/79.2, P=0.003 | ||||||||
| Emotional: 76.3/52.9/65.4, P=0.002 | ||||||||
| 3 | Cheng et al., 2007 (21) | Hong Kong | Solid tumor | CT, RT or CCRT; 88 patients | Prospective, observational | WHO scale | FACT-G | Mean FACT-G score by OM severity slight/moderate/severe/very severe |
| Physical: 8.64/19.00/17.00/11.44, P=0.124 | ||||||||
| Functional: 12.79/11.07/10.14/10.33, P=0.519 | ||||||||
| Emotional: 16.46/16.37/13.93/12.89, P=0.052 | ||||||||
| Social/family: 18.25/15.53/14.21/15.67, P=0.013 (P<0.05) | ||||||||
| 4 | Elting et al. 2008 (22) | Multiple | HNC | RT; 191 patients | Prospective, observational | Patient-reported mouth and throat soreness (MTS) | OMDQ with MTS, FACT-G, FACIT | Mean FACT-G scores at baseline/week 6: |
| Total: 85.1/69; functional well-being domain: 18.3/12.3 | ||||||||
| The reduction in QoL associated with MTS was significant, and there was a correlation between severity of MTS and drop in QoL | ||||||||
| 5 | Cheng et al., 2010 (23) | Hong Kong | Any | CT, RT or CCRT 137 patients | Prospective, observational | WHO scale | OMQoL | Mean OMQoL scores no OM/mild OM (G1 and G2)/severe OM (G3 and G4): |
| Symptom: 97.1/89.3/60, P<0.01 | ||||||||
| Swallowing: 97.6/89.1/52.4, P<0.01 | ||||||||
| Diet: 92.5/82.1/47.9, P<0.01 | ||||||||
| Social: 98.9/93.6/62.1, P<0.01 | ||||||||
| 6 | Kim et al., 2012 (24) | Korea | Solid | CT 322 patients | Prospective, observational | No scale, only symptoms | FACT-G | Mean FACT-G score OM-negative /OM-positive |
| Total: 75.09/70.26, P<0.001 | ||||||||
| Physical: 22.47/19.09, P<0.001 | ||||||||
| Emotional: 17.97/16.74, P<0.001 | ||||||||
| Social/family: 18.04/18.33, P=0.569 | ||||||||
| Educational: 16.55/15.90, P=0.216 | ||||||||
| 7 | Martinez et al. 2014 (25) | Portugal | Lymphoma | 30 patients | Prospective, longitudinal, observational | WHO scale | OMDQ | QoL suffered in eating, drinking and because of pain (P<0.001) |
| Higher the grade of mucositis-severe pain (P<0.001) | ||||||||
| 8 | Sakellari et al. 2015 (26) | Greece | Blood | HSCT; 39 patients | Prospective, observational | WHO scale | FACT-G | Mean total FACT-G score: 78.8±19.1 on day 1, 70.5±21.3 on day +7 (P=0.006) |
| Reduction in physical well-being domain between day 1 (19.6±6.7) and day +7 (14.8±6.8) (P<0.001) | ||||||||
| 9 | Franco et al. 2017 (27) | Italy | HNC | RT, 21 patients | Prospective, longitudinal, observational | OMAS scale | FACT-HN, FACT-G, OMWQ-HN | Mean score: base line/6 weeks |
| FACT-HN: 110.56/89.80 | ||||||||
| FACT-G: 81.70/70.50 | ||||||||
| OMWQ-HN:11.00/24.52 | ||||||||
| 10 | Staudenm-aier et al. 2017 (28) | Germany | Blood | HSCT | Prospective, longitudinal, observational | WHO scale | EORTC, QLQ-C30, QLQ-OH-15 | Mean Score no OM/OM |
| No statistically significant difference in any parameters except | ||||||||
| Physical −7.5±26.3/34.5±25.4 (P<0.05) | ||||||||
| Oral health-related QOL-7.7±11.9/24.3±20.1 (P<0.05) |
CT, chemotherapy, RT, radiotherapy, HNC, head and neck cancer, HSCT, hematopoietic stem cell; MQOLS-CA, Multidimensional Quality of Life scale Cancer version; POMS, Profile of Mood States; EORTC QLQ-C30, European Organization for Research and Treatment of Cancer Core Quality-of-Life Questionnaire; FACIT, Functional Assessment of Chronic Illness Therapy-Fatigue subscale questionnaire; FACT-G, Functional Assessment of Cancer Therapy General score; MTS, patient-reported mouth and throat soreness; OMDQ, Oral Mucositis Daily Questionnaire; OMQoL, OM specific QoL measure; WHO, World Health Organization; OMAS, Oral Mucositis assessment scale; QLQ-OH, Quality-of-Life Questionnaire-Oral Health.
Type of cancers
Majority of the included studies were conducted on patients who were undergoing treatment for solid tumors (four) followed by hematological malignancies (three) and head and neck cancer (two). One study did not categorize the cancer type and considered OM following onco-therapy irrespective of the disease subtype (23).
Assessment of OM and QoL
OM incidence and severity was assessed either by oral examination using the World Health Organization’s Oral Toxicity Scale (WHO-OTS), the Common Terminology Criteria for Adverse Events (CTCAE) v4 scale or through patient self-administered questionnaires like patient-reported mouth and throat soreness (MTS) or Oral Mucositis assessment scale (OMAS). QoL was assessed through validated questionnaires either by a face to face interview or a self-administered method. Functional Assessment of Cancer Therapy (FACT-G) was the most commonly used questionnaire, followed by the Oral Mucositis Daily Questionnaire (OMDQ), European Organization for Research and Treatment of Cancer Core Quality-of-Life Questionnaire (EORTC-QLQ-C30), Multidimensional Quality of Life scale Cancer version (MQoLS-CA), and OM specific QoL measure (OMQoL).
Impact of OM on QoL
All the nine studies which utilized generalized QoL measurements reported reduction in overall QoL with OM; however, disparities could be noted among the various dimensions of QoL among the reported studies. Kim et al. (24) and Dodd et al. (19) reported significant reductions in QoL with alteration in emotional functions such as depression and anger. However, others did not find any significant difference in the emotional state. Four studies reported significant reductions in the physical dimension of QoL (20,24,26,28) whereas three studies reported significant decline in social aspects (20,21,23). Two studies utilized measurements which were specific for oral health and related dimensions affecting QoL including speaking, swallowing and similar attributes (23,25). Cheng et al. identified that mouth and throat pain scores were independent predicators of difficulty in chewing, swallowing and speaking with multiple regressions (23).
Discussion
OM remains one of the most prominent side effect of cancer treatment with no effective intervention yet. Oral mucosal cells replicate quickly and when cell replication becomes inhibited by CT or, the oral mucosa becomes thin and inflamed, mucositis ensues (29).
Even though previously mucositis was investigated in studies which evaluated the impact of cancers of the head and neck region on QoL, it was required to make the assumption that patients’ reports of symptoms including pain and difficulty in swallowing, are related to mucositis (4,30,31). Summaries on the negative impact of mucositis which were based on such inferences had to be dealt with caution as there are other imperative disease- and management-related factors to be considered as well (4). Literature search revealed that very few studies have examined the impact of mucositis on QoL, independent of other disease/treatment problems to explore the potentially intricate relationship between severity of mucositis, patient-reported symptoms, and multiple domains of QoL. We have critically reviewed and summarized the characteristics of those studies which have specifically targeted the impact of OM on various aspects of QoL.
Mucositis can affect each of the major QoL dimensions. Pain and difficulty in swallowing can impact physical wellbeing and the subsequent impairment in diet and communication lead to functional problems that can impact a person’s social well-being. The complex mouth care regimens can also be considered as functional impairment. These penalties may have a substantial impact on emotional well-being due to isolation and loss of social interactions. All the studies reported significant reduction in QoL on one or more of these aforementioned four dimensions on different subscales.
The studies that have evaluated QOL have predominantly used instruments for the overall assessment of cancer treatment with or without added tools of oral health. This is in part due to the lack of a widely accepted instrument during the period of time when these studies were conducted. The most commonly used instrument was the FACT-G (Functional Assessment of Cancer Therapy-General score) which is a multidimensional instrument designed to evaluate the QoL and severity of pain in patients with chronic diseases such as cancer. More recently, questionnaires specially designed for OM were introduced (23,25). Oropharyngeal Mucositis-specific Quality-of-Life (OMQoL) questionnaire include relevant dimensions which include symptomatology, swallowing properties, nutrition and social aspects for estimating QoL in OM patients after cancer therapy. However, more validation studies on different populations are mandatory to establish the efficacy of this QoL instrument. The reported studies were restricted to a few continents with sparse representation from elsewhere on the globe. Distinct dietary habits in different populations according to the lifestyle of an individual and socioeconomic status can also influence the OM symptoms and hence QoL.
Five studies reported the relationship between QoL and severity of mucositis as graded by a physician using a scale whereas other studies inferred the severity with the patient’s reports of symptoms, such as pain and difficulty in swallowing. Reports have suggested concordance between mucositis severity assessed by the physician and patient reported outcomes (32). However, inconsistencies exist between the physician’s evaluation of severity and patient-reported symptoms which may be due to the difference between the ill experience of OM and its corresponding observable manifestations (22,33). This is more likely when mucositis involves sites not easily observed by the clinicians. In addition to the challenges in measuring the severity of the disease there is no consensus on a method for assessing severity of OM though numerous scales which are in use. Moreover, variability in scoring by the physician can also lead to conflicting estimates of severity (34). Precise and robust reporting of OM is critical for implementation of prophylactic and therapeutic measures to improve QoL after CT/RT management. Several studies have identified a correlation between the severity of mucositis and reduction in QoL; this association however was not comparable quantitatively due to difference in cancer types, treatment regimens, chosen time points (during or after therapy) and the instruments used for QoL measurements.
During the most symptomatic phase of OM, high levels of pain and consequent dysfunctions on swallowing, chewing, drinking and speaking has a significant impact on QoL. Opioids are the mainstay analgesics given for the treatment of mucositis (34). The poor pain control in OM and its subsequent impact on QoL reported in these studies highlights the importance of developing management strategies that do more than control the symptoms of mucositis. Prevention of mucositis-induced pain and reduction in its severity are critical in attaining an improvement in patient reported outcomes. A study had reported that the desire for QoL outcomes including less suffering, improved eating and communication ability; vary significantly among patients even though long-term survival is the most desired outcome of any treatment (35). Appraisal of patients’ preferences for the acute and long-term consequences of the aggressive treatment protocols and clarification of the QoL implications could be an important part of clinical decision making, particularly when there is a lack of clear survival advantage of one option versus another (4,34,35). This could be helpful to address the difficulty in treatment-planning owing to the inter-patient variability in preferences.
Implications for future research
Our understanding of the impact of mucositis on QoL would improve by integrating prospective longitudinal evaluation of the severity of OM, symptoms reported, functional status, and QoL of patients into a single study. Such studies would help to the delineate the intricate relationship between physician-graded mucositis patients’ symptomatology, and other domains of QoL. Unscheduled dose reductions or therapy breaks due to severe mucositis may potentially compromise the efficacy of treatment and result in further reduction in quality of life. Treatment expenses for patients with OM are very high due to hospitalization, opioid use, and a greater need for supplementation of nutrition which can have an impact on QoL. Studies that assess the impact of these additional costs on QoL are needed to decrease function loss, minimize symptom burden, and lower treatment costs.
Conclusion
This systematic review addresses the impact of OM on QoL in patients undergoing oncologic treatment. Despite a considerable number of publications, limitations of study design and reporting of QoL continues to limit possibilities for meaningful interpretations. Several assessment tools for OM and QoL are available; a standard option is yet to be established. Overall, we found that the impact of OM on QoL extends beyond the local oral complications and has been shown to affect the functioning domains like the physical, emotional, and psychological aspects. Further prospective, longitudinal and, ideally, randomized QoL data are awaited to support the findings of our review.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Shankargouda Patil, Sachin C. Sarode and Kamran Awan) for the series “Oral Pre-cancer and Cancer” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.02.77). The series “Oral Pre-cancer and Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25 676 887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 2015;385:977-1010. [Crossref] [PubMed]
- Cella DF, Cherin EA. Quality of life during and after cancer treatment. Compr Ther 1988;14:69-75. [PubMed]
- Mazzotti E, Antonini Cappellini GC, Buconovo S, et al. Treatment-related side effects and quality of life in cancer patients. Support Care Cancer 2012;20:2553-7. [Crossref] [PubMed]
- Peterman A, Cella D, Glandon G, et al. Mucositis in head and neck cancer: economic and quality-of-life outcomes. J Natl Cancer Inst Monogr 2001;45-51. [Crossref] [PubMed]
- Hoaglin DC, Hawkins N, Jansen JP, et al. Conducting Indirect-Treatment-Comparison and Network-Meta-Analysis Studies: Report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices-Part 2. 2011.
- Sonis ST. Mucositis as a biological process: a new hypothesis for the development of chemotherapy-induced stomatotoxicity. Oral Oncol 1998;34:39-43. [Crossref] [PubMed]
- Sonis ST. New thoughts on the initiation of mucositis. Oral Dis 2010;16:597-600. [Crossref] [PubMed]
- Keefe DM, Gibson RJ. Mucosal injury from targeted anti-cancer therapy. Support Care Cancer 2007;15:483-90. [Crossref] [PubMed]
- Rubenstein EB, Peterson DE, Schubert M, et al. Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Cancer 2004;100:2026-46. [Crossref] [PubMed]
- Curra M, Alberto L, Soares V, et al. Protocolos quimioterápicos e incidência de mucosite bucal. Rev Integr 2018;16:1-9.
- Shirai K, Saitoh J, Musha A, et al. Prospective observational study of carbon-ion radiotherapy for non-squamous cell carcinoma of the head and neck. Cancer Sci 2017;108:2039-44. [Crossref] [PubMed]
- Genot-Klastersky MT, Klastersky J, Awada F, et al. The use of low-energy laser (LEL) for the prevention of chemotherapy- and/or radiotherapy-induced oral mucositis in cancer patients : results from two prospective studies. Support Care Cancer 2008;16:1381-7. [Crossref] [PubMed]
- Scully C, Sonis S, Diz PD. Oral mucositis. Oral Dis 2006;12:229-41. [Crossref] [PubMed]
- Jensen SB, Peterson DE. Oral mucosal injury caused by cancer therapies: current management and new frontiers in research. J Oral Pathol Med 2014;43:81-90. [Crossref] [PubMed]
- Peterson DE, Ohrn K, Bowen J, et al. Systematic review of oral cryotherapy for management of oral mucositis caused by cancer therapy. Support Care Cancer 2013;21:327-32. [Crossref] [PubMed]
- Sonis ST, Elting LS, Keefe D, et al. Perspectives on cancer therapyinduced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 2004;100:1995-2025. [Crossref] [PubMed]
- Worthington HV, Clarkson JE, Bryan G, et al. Interventions for preventing oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev 2011;CD000978. [PubMed]
- Clarkson JE, Worthington HV, Furness S, et al. Interventions for treating oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev 2010;CD001973. [PubMed]
- Dodd MJ, Dibble S, Miaskowski C, et al. A comparison of the affective state and quality of life of chemotherapy patients who do and do not develop chemotherapy-induced oral mucositis. J Pain Symptom Manage 2001;21:498-505. [Crossref] [PubMed]
- Epstein JB, Beaumont JL, Gwede CK, et al. Longitudinal evaluation of the oral mucositis weekly questionnaire-head and neck cancer, a patient-reported outcomes questionnaire. Cancer 2007;109:1914-22. [Crossref] [PubMed]
- Cheng KKF. Oral mucositis and quality of life of Hong Kong Chinese patients with cancer therapy. Eur J Oncol Nurs 2007;11:36-42. [Crossref] [PubMed]
- Elting LS, Keefe DM, Sonis ST, et al. Patient-reported measurements of oral mucositis in head and neck cancer patients treated with radiotherapy with or without chemotherapy: demonstration of increased frequency, severity, resistance to palliation, and impact on quality of life. Cancer 2008;113:2704-13. [Crossref] [PubMed]
- Cheng KKF, Leung SF, Liang RHS, et al. Severe oral mucositis associated with cancer therapy: impact on oral functional status and quality of life. Support Care Cancer 2010;18:1477-85. [Crossref] [PubMed]
- Kim JW, Cha Y, Kim SJ, et al. Association of oral mucositis with quality of life and symptom clusters in patients with solid tumors receiving chemotherapy. Support Care Cancer 2012;20:395-403. [Crossref] [PubMed]
- Martinez JM, Pereira D, Chacim S, et al. Mucositis care in acute leukemia and non-Hodgkin lymphoma patients undergoing high-dose chemotherapy. Support Care Cancer 2014;22:2563-9. [Crossref] [PubMed]
- Sakellari I, Angelopoulou M, Tsopra O, et al. A prospective study of incidence, clinical and quality of life consequences of oral mucositis post palifermin prophylaxis in patients undergoing high-dose chemotherapy and autologous hematopoietic cell transplantation. Ann Hematol 2015;94:1733-40. [Crossref] [PubMed]
- Franco P, Martini S, Di Muzio J, et al. Prospective assessment of oral mucositis and its impact on quality of life and patient-reported outcomes during radiotherapy for head and neck cancer. Med Oncol 2017;34:81. [Crossref] [PubMed]
- Staudenmaier T, Cenzer I, Crispin A, et al. Burden of oral mucositis in stem cell transplant patients-the patients’ perspective. Support Care Cancer 2018;26:1577-84. [PubMed]
- Scully C, Epstein J, Sonis S. Oral mucositis a challenging complication of radiotherapy, chemotherapy, and radiochemotherapy: part 1, pathogenesis and prophylaxis of mucositis. Head Neck 2003;25:1057-70. [Crossref] [PubMed]
- Taphoorn MJ, Henriksson R, Bottomley A, et al. Health-related quality of life in a randomized phase iii study of bevacizumab, temozolomide, and radiotherapy in newly diagnosed glioblastoma. J Clin Oncol 2015;33:2166-75. [Crossref] [PubMed]
- Franzén L, Henriksson R, Littbrand B, et al. Effects of sucralfate on mucositis during and following radiotherapy of malignancies in the head and neck region. A double-blind placebo-controlled study. Acta Oncol 1995;34:219-23. [Crossref] [PubMed]
- Stiff PJ, Erder H, Bensinger WI, et al. Reliability and validity of a patient self-administered daily questionnaire to assess impact of oral mucositis (OM) on pain and daily functioning in patients undergoing autologous hematopoietic stem cell transplantation (HSCT). Bone Marrow Transplant 2006;37:393-401. [Crossref] [PubMed]
- Fromme EK, Eilers KM, Mori M, et al. How accurate is clinician reporting of chemotherapy adverse events? A comparison with patient reported symptoms from the Quality -of Life Questionnaire C30. J Clin Oncol 2004;22:3485-90. [Crossref] [PubMed]
- Lalla RV, Sonis ST, Peterson DE. Management of oral mucositis in patients who have cancer. Dent Clin North Am 2008;52:61-77. [Crossref] [PubMed]
- Shrestha A, Martin C, Burton M, et al. Quality of life versus length of life considerations in cancer patients: A systematic literature review. Psychooncology 2019;28:1367-80. [Crossref] [PubMed]