
Sensitization of tumor cells to cancer therapy by molecularly targeted inhibition of the inhibitor of nuclear factor κB kinase
Introduction
The inhibitor of nuclear factor κB kinase (IKK)-nuclear factor κB (NFκB) pathway is one of the most important cellular signal transduction pathways (1). It consists of members of the NFκB family and the family of inhibitors of NFκB (IκB), the IκB kinase (IKK) complex, and various other regulatory components. The NFκB family includes RelA (p65), RelB, c-Rel, NFκB1/p105 (p50 precursor), and NFκB2/p100 (p52 precursor); the IκB family consists of IκBα, IκBβ, IκBε, Bcl-3, p100/IκBδ, and p105/IκBγ; and the IKK complex is composed of two catalytic subunits, IKKα and IKKβ, and the regulatory subunit IKKγ. Normally, members of the NFκB family form a heterodimer/homodimer that resides in the cytoplasm as an inactive complex in association with a member of the IκB family. Upon stimulation with a stimulus, the so-called canonical or classical pathway is activated, leading to the activation of IKK complex. Activated IKKα and/or IKKβ phosphorylate IκBα at S-32 and S-36. This causes IκBα ubiquitination and degradation by the 26S proteasome, thereby, allowing NFκB to translocate into the nucleus to regulate NFκB target genes. Alternatively, NFκB can be activated through the non-canonical pathway in which some NFκB stimuli can induce IKKα activation via NFκB-inducing kinase, resulting in the formation of p52 after p100 is phosphorylated by the activated IKKα and degraded by the proteasome via the ubiquitin-dependent process. Through regulation of its target genes, NFκB can regulate various physiologic processes such as cell proliferation, migration and survival. Its dysregulation has been implicated in carcinogenesis and tumor development and progression (2-5).
In addition, an increasing body of evidence suggests that activation of the IKK-NFκB pathway also plays a pivotal role in the development of cancer resistance to ionizing radiation (IR) and chemotherapy (2,4,6,7). This is because IR and many chemotherapeutic agents can activate NFκB through the atypical NFκB activation pathway by induction of DNA double-strand breaks (DSBs) (8,9). Activation of the IKK-NFκB pathway renders many types of tumor cells more resistant to IR and chemotherapy presumably via induction of anti-apoptotic proteins (2,4,6,7). Therefore, inhibition of the NFκB transcriptional activity has been extensively exploited as a novel approach to sensitize cancers to radiotherapy and chemotherapy, but has achieved mixed results (2,4,6,7,10). However, some more recent studies provide new insights into the mechanisms whereby activation of the IKK-NFκB pathway increases tumor cell resistance to IR and chemotherapy. These new developments could make a molecular targeted inhibition of the IKK-NFκB pathway more effective in sensitizing tumor cells to cancer therapy.
Activation of the IKK-NFκB pathway by radiotherapy and chemotherapy
IR and various chemotherapeutic drugs are potent DNA damage agents. Exposure of cells to IR and chemotherapeutic agents such as camptothecin (CT), etoposide, or doxorubincin (DOX) induces DSBs. As shown in Figure 1, DSBs stimulate poly(ADP-ribose) polymerase-1 (PARP-1) and the kinase ataxia telangiectasia mutated (ATM). PARR-1 recruits nuclear IKKγ, the E3 ligase PIASy (protein inhibitor of activated STAT Y) and the activated ATM into a complex to facilitate IKKγ sumoylation and phosphorylation by PIASy and ATM consecutively and then IKKγ mono-ubiquitilation by a yet unidentified E3 ligase (11,12). The post-translationally modified IKKγ and activated ATM are then exported from the nucleus to the cytoplasma (8,9). In the cytoplasma, ATM functions as a scaffold protein to aid the assembling of the signalosomes consisting of the ubiquitin-conjugating enzyme UBC13, the E3 ligase tumor necrosis factor receptor-associated factor 6 (TRAF6) and cellular inhibitor of apoptosis protein 1 (cIAP1) or UBC13, the E3 ligase X-linked inhibitor of apoptosis protein (XIAP) and ELKS (protein rich in glutamate, leucine, lysine, and serine) in a stimulus-dependent manner. In the signalosomes, TRAF6 undergoes auto-ubiquitilation to recruits transforming growth factor β (TGFβ)-activated kinase1 (TAK1) and the TAK1-binding proteins TAB2 into the IKK complex composed of IKKα, IKKβ and IKKγ (11,12). Alternatively, ELKS is ubiquitilated by XIAP, which in turn promotes the formation of TAB2-TAK1 and IKKα/β/γ complexes (11,12). The formation of these signalosomes facilitate TAK1 auto-phosphorylation and IKKβ trans-phosphorylation by TAK1, leading to the activation of IKKβ (11,12). The activated IKKβ phosphorylates IκB to induce its ubiquitilation and then degradation by the 26S proteasome, which releases NFκB for nuclear translocation to initiate the transcription of NFκB target genes (1,9,13). In addition, activated IKKβ can also regulate various cellular functions in an NFκB-independent manner (14,15). Both NFκB-dependent and independent effects of IKKβ can contribute to tumor resistance to cancer therapy as discussed below.
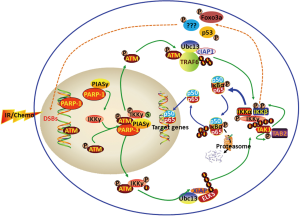
NFκB-dependent effects
NFκB is a transcriptional factor that binds to specific DNA sequences in target genes, designated as κB-elements. Most κB-elements are 10 bp in length with the consensus sequence 5'-GGGRNWYYCC-3', where R denotes a purine base, N means any base, W stands for an adenine or thymine, and Y represents a pyrimidine base (16,17). There are more than 400 genes that contain κB-elements and their expression can be regulated by NFκB but in a cell-type specific and a cell context-dependent manner (7,10). Among these NFκB-targeted genes, a number of them are anti-apoptotic genes including bcl-2, bcl-xL, survivin, and XIAP. Increased expression of these genes induced by NFκB activation has been implicated in radioresistance and chemoresistance in a wide variety of tumor cells. As such, inhibition of the NFκB transcriptional activity has been extensively exploited as a novel approach to sensitize cancers to radiotherapy and chemotherapy (2,4,6,7). For example, HT1080 human fibrosarcoma cells expressing a super-repressor form of IκBα were more sensitive to daunorubicin and IR-induced apoptosis than the wild-type HT1080 cells (18). Inhibition of NFκB activity using an NFκB decoy reduced chemoresistance of human stomach cancer cell line to 5-fluorouracil treatment (19). The sensitivity of breast cancer cells to paclitaxel was enhanced by IκBα super-repressor and parthenolide that inhibited the constitutive NFκB activity in the cells (20). Treatment of resistant Capan-1 and 818-4 pancreatic cancer cells with various NFκB inhibitors or transfection of the cells with an IκBα super-repressor increased the induction of apoptosis by etoposide or doxorubincin (DOX) (21). Down-regulation of RelA by RNAi sensitized HCT116 colon cancer cells to CPT-11 (22). Inhibition of NFκB activity increased the cisplatin-induced apoptosis in the cisplatin-resistant Caov-3 ovarian cancer cells not only in vitro but also in vivo (23). Curcumin potentiated the antitumor activity of gemcitabine in an orthotopic pancreatic cancer model in part via inhibition of NFκB-regulated gene expression (24). Targeted inhibition of NFκB with a RNA aptamer reduced tumor resistance to Dox in A549 human non-small cell lung cancer cells both in vitro and in vivo (25). The list of publications demonstrating that NFκB inhibition using a variety of inhibitors sensitizes various types of tumor cells to the induction of apoptosis by different chemotherapeutic agents are still growing (2,4-7,10). Similarly, inhibition of NFκB also increased apoptosis of various types of cancer cells induced by IR (2,4-7,10). It was reported that human malignant glioma cell lines overexpressing IκBα were more sensitive than the parental cells to IR (26). Expression of a dominant negative IκBα in Hela cells increased their sensitivity to IR-induced cytotoxicity (27). Inhibition of NFκB activation with PS-341 or infection with an adenovirus encoding IκBα super-repressor increased IR-induced apoptosis and enhanced radiosensitivity in colorectal cancer cells in vitro and in vivo (28). Human squamous carcinoma SCC-35 cells stably expressing a truncated human RelA exhibited a deficiency in radiation-induced NFκB activation and a higher sensitivity to radiation-induced apoptosis (29). Curcumin also potentiated the antitumor effects of IR in HCT 116 colorectal cancer xenografts in nude mice by suppressing NFκB and NFκB-regulated gene products (30). However, not all tumor cells are killed by IR and chemotherapy through induction of apoptosis. Some die via induction of mitotic cell death or senescence after exposure to a chemotherapeutic agent and/or IR (31,32). Furthermore, other studies showed that activation of NFκB sometimes played a pro-apoptotic role in certain conditions (33,34). This is because activation of NFκB can also up-regulate the expression of the pro-apoptotic death receptors DR4, DR5, Fas and Fas ligand in a drug-specific and cell type-dependent manner (33-36). Therefore, recent studies have been focused on the identification of the upstream components of the IKK-NFκB pathway, in which inhibition can sensitize tumor cells to radiotherapy and chemotherapy in both apoptosis-dependent and apoptosis-independent manners.
NFκB-independent effects
Although IKKβ plays an essential role in NFκB activation induced by various cancer therapies via induction of IκB phosphorylation, ubiquitilation and degradation, it has many other NFκB-independent functions (14,15). Some of these functions have been implicated in regulation of tumor cell sensitivity to IR and chemotherapy. For example, IKKβ can phosphorylate the tumor suppressor Foxo3a and consequently induces Foxo3a nuclear exclusion and degradation, thereby inhibiting Foxo3a-mediated transcription of genes encoding molecules that can promote cell-cycle arrest and apoptosis (37,38). Therefore, inhibition of IKKβ can increase Foxo3a anti-tumor function. In addition, it has been shown that IKKβ can directly phosphorylate Aurora kinase A to regulate its stability for the maintenance of bipolar sindle assembly and genomic stability (39). However, a recent study showed that inhibition of IKKβ with a specific inhibitor affects cell cycle progression at multiple positions without direct inhibition of various mitotic kinases including cyclin-dependent kinase 1, Aurora A and B, polo-like kinase 1, and NIMA (never in mitosis gene a)-related kinase 2 (40). Therefore, the mechanisms by which IKKβ regulates cell cycle progression have yet to be determined. Furthermore, IKKβ can phosphorylate p53 at serines 362 and 366 which leads to p53 ubiquitilation and degradation by β-tranducin repeat-containing protein in an Mdm2-independent manner (41). This suggests that IKKβ inhibition can stabilize p53 to induce tumor cell cycle arrest and/or apoptosis.
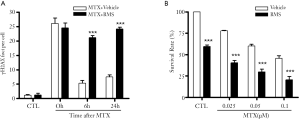
It has been well established that IR and many chemotherapeutic drugs kill cancer cells primarily by induction of DSBs and efficient repair of DSBs is required for the clonogenic survival of the cells exposed to IR and chemotherapeutic agents (42,43). Therefore, in our recent studies we examined whether activation of the IKK-NFκB pathway by IR can promote cancer cell survival in part by regulating the repair of DSBs in an IKKβ-dependent but NFκB-independent manner (44). We first used BMS-345541 (BMS), a specific IKKβ inhibitor (45), to selectively inhibit the IKK-NFκB pathway and found that it could significantly inhibit the repair of IR-induced DSBs in MCF-7 human breast cancer cells and H1299 and H1648 human lung cancer cells. Interestingly, selective inhibition of the NFκB transcriptional activity by ectopical expression of a mutant IκBα or down-regulation of RelA by RNAi had no such effect. The repair of DSBs was also not affected by down-regulation of IKKα expression with IKKα shRNA, but was significantly inhibited by silencing IKKβ expression with IKKβ shRNA. Similar findings were also observed in IKKα and/or IKKβ knockout mouse embryonic fibroblasts. More importantly, inhibition of IKKβ with an inhibitor or down-regulation of IKKβ with IKKβ shRNA sensitized MCF-7 cells to IR-induced clonogenic cell death in an apoptosis-independent manner. DSB repair function and resistance to IR were completely restored by IKKβ reconstitution in IKKβ-knockdown MCF-7 cells. These findings demonstrate that IKKβ regulates the repair of DSBs and inhibition of IKKβ activity can sensitize cancer cells to IR at least in part via inhibition of DSB repair. As such, specific inhibition of IKKβ may represents a more effective approach to sensitize cancer cells to radiotherapy. In addition, our preliminary studies also show that IKKβ inhibition by BMS can suppress the repair of DSBs induced not only by IR but also by the chemotherapeutic agent methotrexate (MTX) (Figure 2). Inhibition of DSB repair by BMS also led to sensitization of MCF-7 cells to MTX. Therefore, IKKβ inhibitors such as BMS have the potential to be used as tumor sensitizers for chemotherapy as well.
However, the mechanisms by which IKKβ regulates DSB repair have yet to be elucidated. Although our data showed that IKKβ can regulate the repair of DSBs independent of the NFκB-RelA transcriptional activity, it remains to be determined if activation of the other members of the NFκB family by IKKβ, such as c-Rel, may be involved in the regulation of DSB repair. For example, a recent report showed that activation of IKKβ up-regulates the expression of Claspin via c-Rel (46). Claspin can regulate DNA damage-activated checkpoint response by promoting ataxia telangiectasia and Rad3-related protein (ATR)-mediated Chk1 phosphorylation and activation (39,47). However, it is not unexpected to find that IKKβ may regulate DSB repair independent of NFκB, because several non-IκB targets of IKKβ have been identified recently and their numbers are rising (14,48). For example, it has been shown that IKKβ can directly phosphorylate Aurora kinase A to regulate its stability for the maintenance of bipolar sindle assembly and genomic stability (39). Particularly, a recent study showed that IKKβ translocates to the nucleus following UV irradiation (49). It is plausible that IKKβ may enter the nucleus following IR treatments to directly regulate the DSB repair processes. Alternatively, it will be interesting to determine if IKKβ-dependent DSB repair could be initiated by a mechanism involving the cytoplasmic IKKβ-ATM axis (8,9,50). Identification of IKKβ substrate(s) required for DSB repair and elucidation of the mechanisms by which IKKβ regulates DSB repair will uncover novel molecular targets for sensitization of tumor cells to cancer therapy with IR and chemotherapeutic drugs in the future.
Sensitizing tumor cells to cancer therapy by molecularly targeted inhibition of IKKβ
As discussed above, molecularly targeted inhibition of IKKβ can inhibit both NFκB-dependent and -independent effects and sensitize tumor cells to IR and chemotherapy in apoptosis-dependent and -independent manners. Therefore, IKKβ has emerged as a better target than other components in the IKK-NFκB pathway for developing novel tumor sensitizers and substantial efforts have been devoted to the development of highly specific IKKβ inhibitors (5,10,51). Based on the mechanism of action, the known IKKβ inhibitors can be divided into three categories: adenosine triphosphate (ATP) analogs; allosteric inhibitors; and thiol-reactive compounds. ATP analogs include β-carboline natural products and derivatives such as PS-1145 and ML120B (5,51-53). BMS is a representative of allosteric IKKβ inhibitors (45,51). Thiol-reactive compounds that interact with IKKβ at Cys-179 include parthenolide and arsenite (54,55). All these compounds are highly specific toward IKKβ except thiol-reactive compounds. It was reported that PS-1145 and ML120B exhibited strong antitumor activities against multiple myeloma, diffuse large B-cell lymphoma, chronic myelogenous leukemia, and prostate cancer in several preclinical studies (56-58). BMS exerted an antitumor activity in a melanoma xenograft model by inducing melanoma cell apoptosis (59). It is likely that these compounds have the potential to be used as tumor sensitizers to enhance tumor cell response to IR and chemotherapy by selectively inhibiting the activation of the IKK-NFκB pathway. This suggestion is supported by our recent in vitro study as discussed previously, in which we found that BMS increased IR- and MTX-induced tumor cell killing in part by inhibition of the repair of DSBs (44). It remains to be determined if IKKβ inhibitors can also sensitize tumor cells to cancer therapy in vivo.
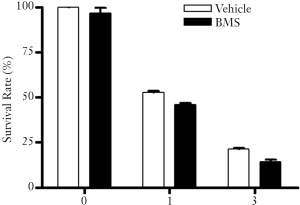
Interestingly, even though BMS is cytotoxic to some tumor cells and can sensitize MCF-7 human breast cancer cells to IR and MTX, it is a relatively safe agent that does not cause noticeable normal tissue damage in vivo (60-62). Moreover, we have found that BMS does not adversely affect the clonogenic survival of mouse bone marrow hematopoietic progenitor cells in vitro with or without exposure to IR at a concentration (5 μM) that is cytotoxic to MCF-7 cells and can sensitize the tumor cells to IR (Figure 3). However, extra caution has to be exercised to ensure that IKKβ inhibition with a potent inhibitor will not cause overly adverse effects before IKKβ inhibitors can be tested in clinic for cancer treatment, because inhibition of the IKK-NFκB pathway can compromise patients’ immune systems. This risk can be mitigated by short treatment with the inhibitors to avoid long-term immunosuppression. Overcoming this and other potential health risks of inhibition of the IKK-NFκB pathway will make IKKβ inhibitors a potential anti-tumor agent and a better tumor sensitizer.
Acknowledgments
The authors apologize to those whose contributions were not referred because of the limitation of space for the review.
Funding: This manuscript was supported in part by the National Institutes of Health (NIH) (R01CA86688 and R01CA122023), the Winthrop W. Rockefeller Endowment for Leukemia Research and the Arkansas Research Alliance Scholarship from the Arkansas Science & Technology Authority.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2012.05.04). DZ serves as an unpaid editorial board member of Translational Cancer Research. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol 2007;8:49-62. [PubMed]
- Kim HJ, Hawke N, Baldwin AS. NF-kappaB and IKK as therapeutic targets in cancer. Cell Death Differ 2006;13:738-47. [PubMed]
- Luo JL, Kamata H, Karin M. IKK/NF-kappaB signaling: balancing life and death--a new approach to cancer therapy. J Clin Invest 2005;115:2625-32. [PubMed]
- Nakanishi C, Toi M. Nuclear factor-kappaB inhibitors as sensitizers to anticancer drugs. Nat Rev Cancer 2005;5:297-309. [PubMed]
- Van Waes C. Nuclear factor-kappaB in development, prevention, and therapy of cancer. Clin Cancer Res 2007;13:1076-82. [PubMed]
- Baud V, Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov 2009;8:33-40. [PubMed]
- Li F, Sethi G. Targeting transcription factor NF-kappaB to overcome chemoresistance and radioresistance in cancer therapy. Biochim Biophys Acta 2010;1805:167-80.
- Wu ZH, Shi Y, Tibbetts RS, et al. Molecular linkage between the kinase ATM and NF-kappaB signaling in response to genotoxic stimuli. Science 2006;311:1141-6. [PubMed]
- Wu ZH, Miyamoto S. Many faces of NF-kappaB signaling induced by genotoxic stress. J Mol Med 2007;85:1187-202. [PubMed]
- Gupta SC, Sundaram C, Reuter S, et al. Inhibiting NF-kappaB activation by small molecules as a therapeutic strategy. Biochim Biophys Acta 2010;1799:775-87.
- Hinz M, Stilmann M, Arslan SC, et al. A cytoplasmic ATM-TRAF6-cIAP1 module links nuclear DNA damage signaling to ubiquitin-mediated NF-kappaB activation. Mol Cell 2010;40:63-74. [PubMed]
- Wu ZH, Wong ET, Shi Y, et al. ATM- and NEMO-dependent ELKS ubiquitination coordinates TAK1-mediated IKK activation in response to genotoxic stress. Mol Cell 2010;40:75-86. [PubMed]
- Israël A. The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harb Perspect Biol 2010;2:a000158 [PubMed]
- Chariot A. The NF-kappaB-independent functions of IKK subunits in immunity and cancer. Trends Cell Biol 2009;19:404-13. [PubMed]
- Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-kappaB signaling pathways. Nat Immunol 2011;12:695-708. [PubMed]
- Sen R, Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell 1986;47:921-8. [PubMed]
- Chen FE, Ghosh G. Regulation of DNA binding by Rel/NF-kappaB transcription factors: structural views. Oncogene 1999;18:6845-52. [PubMed]
- Wang CY, Mayo MW, Baldwin AS Jr. TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science 1996;274:784-7. [PubMed]
- Uetsuka H, Haisa M, Kimura M, et al. Inhibition of inducible NF-kappaB activity reduces chemoresistance to 5-fluorouracil in human stomach cancer cell line. Exp Cell Res 2003;289:27-35. [PubMed]
- Patel NM, Nozaki S, Shortle NH, et al. Paclitaxel sensitivity of breast cancer cells with constitutively active NF-kappaB is enhanced by IkappaBalpha super-repressor and parthenolide. Oncogene 2000;19:4159-69. [PubMed]
- Arlt A, Vorndamm J, Breitenbroich M, et al. Inhibition of NF-kappaB sensitizes human pancreatic carcinoma cells to apoptosis induced by etoposide (VP16) or doxorubicin. Oncogene 2001;20:859-68. [PubMed]
- Guo J, Verma UN, Gaynor RB, et al. Enhanced chemosensitivity to irinotecan by RNA interference-mediated down-regulation of the nuclear factor-kappaB p65 subunit. Clin Cancer Res 2004;10:3333-41. [PubMed]
- Mabuchi S, Ohmichi M, Nishio Y, et al. Inhibition of NFkappaB increases the efficacy of cisplatin in in vitro and in vivo ovarian cancer models. J Biol Chem 2004;279:23477-85. [PubMed]
- Kunnumakkara AB, Guha S, Krishnan S, et al. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res 2007;67:3853-61. [PubMed]
- Mi J, Zhang X, Rabbani ZN, et al. RNA aptamer-targeted inhibition of NF-kappa B suppresses non-small cell lung cancer resistance to doxorubicin. Mol Ther 2008;16:66-73. [PubMed]
- Yamagishi N, Miyakoshi J, Takebe H. Enhanced radiosensitivity by inhibition of nuclear factor kappa B activation in human malignant glioma cells. Int J Radiat Biol 1997;72:157-62. [PubMed]
- Bradbury CM, Markovina S, Wei SJ, et al. Indomethacin-induced radiosensitization and inhibition of ionizing radiation-induced NF-kappaB activation in HeLa cells occur via a mechanism involving p38 MAP kinase. Cancer Res 2001;61:7689-96. [PubMed]
- Russo SM, Tepper JE, Baldwin AS Jr, et al. Enhancement of radiosensitivity by proteasome inhibition: implications for a role of NF-kappaB. Int J Radiat Oncol Biol Phys 2001;50:183-93. [PubMed]
- Kim KM, Zhang Y, Kim BY, et al. The p65 subunit of nuclear factor-kappaB is a molecular target for radiation sensitization of human squamous carcinoma cells. Mol Cancer Ther 2004;3:693-8. [PubMed]
- Kunnumakkara AB, Diagaradjane P, Guha S, et al. Curcumin sensitizes human colorectal cancer xenografts in nude mice to gamma-radiation by targeting nuclear factor-kappaB-regulated gene products. Clin Cancer Res 2008;14:2128-36. [PubMed]
- Ewald JA, Desotelle JA, Wilding G, et al. Therapy-induced senescence in cancer. J Natl Cancer Inst 2010;102:1536-46. [PubMed]
- Roninson IB, Broude EV, Chang BD. If not apoptosis, then what? Treatment-induced senescence and mitotic catastrophe in tumor cells. Drug Resist Updat 2001;4:303-13. [PubMed]
- Jin F, Liu X, Zhou Z, et al. Activation of nuclear factor-kappaB contributes to induction of death receptors and apoptosis by the synthetic retinoid CD437 in DU145 human prostate cancer cells. Cancer Res 2005;65:6354-63. [PubMed]
- Campbell KJ, Rocha S, Perkins ND. Active repression of antiapoptotic gene expression by RelA(p65) NF-kappa B. Mol Cell 2004;13:853-65. [PubMed]
- Hsu SC, Gavrilin MA, Lee HH, et al. NF-kappa B-dependent Fas ligand expression. Eur J Immunol 1999;29:2948-56. [PubMed]
- Feng G, Li Y, Bai Y. Induction of Fas receptor and Fas ligand by nodularin is mediated by NF-kappaB in HepG2 cells. Toxicol Appl Pharmacol 2011;251:245-52. [PubMed]
- Hu MC, Lee DF, Xia W, et al. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell 2004;117:225-37. [PubMed]
- Tezil T, Bodur C, Kutuk O, et al. IKK-beta mediates chemoresistance by sequestering FOXO3; A critical factor for cell survival and death. Cell Signal 2012;24:1361-8. [PubMed]
- Irelan JT, Murphy TJ, DeJesus PD, et al. A role for IkappaB kinase 2 in bipolar spindle assembly. Proc Natl Acad Sci USA 2007;104:16940-45. [PubMed]
- Blazkova H, von Schubert C, Mikule K, et al. The IKK inhibitor BMS-345541 affects multiple mitotic cell cycle transitions. Cell Cycle 2007;6:2531-40. [PubMed]
- Xia Y, Padre RC, De Mendoza TH, et al. Phosphorylation of p53 by IkappaB kinase 2 promotes its degradation by beta-TrCP. Proc Natl Acad Sci USA 2009;106:2629-34. [PubMed]
- Helleday T, Petermann E, Lundin C, et al. DNA repair pathways as targets for cancer therapy. Nat Rev Cancer 2008;8:193-204. [PubMed]
- Martin SA, Lord CJ, Ashworth A. DNA repair deficiency as a therapeutic target in cancer. Curr Opin Genet Dev 2008;18:80-6. [PubMed]
- Wu L, Shao L, An N, et al. IKKbeta regulates the repair of DNA double-strand breaks induced by ionizing radiation in MCF-7 breast cancer cells. PLoS One 2011;6:e18447 [PubMed]
- Burke JR, Pattoli MA, Gregor KR, et al. BMS-345541 is a highly selective inhibitor of I kappa B kinase that binds at an allosteric site of the enzyme and blocks NF-kappa B-dependent transcription in mice. J Biol Chem 2003;278:1450-6. [PubMed]
- Kenneth NS, Mudie S, Rocha S. IKK and NF-kappaB-mediated regulation of Claspin impacts on ATR checkpoint function. EMBO J 2010;29:2966-78. [PubMed]
- Saeki T, Ouchi M, Ouchi T. Physiological and oncogenic Aurora-A pathway. Int J Biol Sci 2009;5:758-62. [PubMed]
- Suzuki K, Verma IM. Phosphorylation of SNAP-23 by IkappaB kinase 2 regulates mast cell degranulation. Cell 2008;134:485-95. [PubMed]
- Tsuchiya Y, Asano T, Nakayama K, et al. Nuclear IKKbeta is an adaptor protein for IkappaBalpha ubiquitination and degradation in UV-induced NF-kappaB activation. Mol Cell 2010;39:570-82. [PubMed]
- Ahmed KM, Li JJ. ATM-NF-kappaB connection as a target for tumor radiosensitization. Curr Cancer Drug Targets 2007;7:335-42. [PubMed]
- Lee DF, Hung MC. Advances in targeting IKK and IKK-related kinases for cancer therapy. Clin Cancer Res 2008;14:5656-62. [PubMed]
- Castro AC, Dang LC, Soucy F, et al. Novel IKK inhibitors: beta-carbolines. Bioorg Med Chem Lett 2003;13:2419-22. [PubMed]
- Wen D, Nong Y, Morgan JG, et al. A selective small molecule IkappaB Kinase beta inhibitor blocks nuclear factor kappaB-mediated inflammatory responses in human fibroblast-like synoviocytes, chondrocytes, and mast cells. J Pharmacol Exp Ther 2006;317:989-1001. [PubMed]
- Kapahi P, Takahashi T, Natoli G, et al. Inhibition of NF-kappa B activation by arsenite through reaction with a critical cysteine in the activation loop of Ikappa B kinase. J Biol Chem 2000;275:36062-6. [PubMed]
- Kwok BH, Koh B, Ndubuisi MI, et al. The anti-inflammatory natural product parthenolide from the medicinal herb Feverfew directly binds to and inhibits IkappaB kinase. Chem Biol 2001;8:759-66. [PubMed]
- Cilloni D, Messa F, Arruga F, et al. The NF-kappaB pathway blockade by the IKK inhibitor PS1145 can overcome imatinib resistance. Leukemia 2006;20:61-7. [PubMed]
- Yemelyanov A, Gasparian A, Lindholm P, et al. Effects of IKK inhibitor PS1145 on NF-kappaB function, proliferation, apoptosis and invasion activity in prostate carcinoma cells. Oncogene 2006;25:387-98. [PubMed]
- Lam LT, Davis RE, Pierce J, et al. Small molecule inhibitors of IkappaB kinase are selectively toxic for subgroups of diffuse large B-cell lymphoma defined by gene expression profiling. Clin Cancer Res 2005;11:28-40. [PubMed]
- Yang J, Amiri KI, Burke JR, et al. BMS-345541 targets inhibitor of kappaB kinase and induces apoptosis in melanoma: involvement of nuclear factor kappaB and mitochondria pathways. Clin Cancer Res 2006;12:950-60. [PubMed]
- Gillooly KM, Pattoli MA, Taylor TL, et al. Periodic, partial inhibition of IkappaB Kinase beta-mediated signaling yields therapeutic benefit in preclinical models of rheumatoid arthritis. J Pharmacol Exp Ther 2009;331:349-60. [PubMed]
- Townsend RM, Postelnek J, Susulic V, et al. A highly selective inhibitor of IkappaB kinase, BMS-345541, augments graft survival mediated by suboptimal immunosuppression in a murine model of cardiac graft rejection. Transplantation 2004;77:1090-4. [PubMed]
- McIntyre KW, Shuster DJ, Gillooly KM, et al. A highly selective inhibitor of I kappa B kinase, BMS-345541, blocks both joint inflammation and destruction in collagen-induced arthritis in mice. Arthritis Rheum 2003;48:2652-9. [PubMed]


