
Molecular targeting of low-grade serous and mucinous carcinomas of the ovary or peritoneum
Introduction
Historically, all women with epithelial ovarian cancer or primary peritoneal cancer, regardless of their tumor’s histologic subtype, have been treated similarly within single-institution, investigator-initiated, or cooperative group trials. However, within the past few years, based on our enhanced understanding of the heterogeneity of ovarian or peritoneal cancer related to refinement of pathologic criteria, elucidation of molecular biology, and reports of clinical behavior, separate clinical trials for specific subtypes have been developed and conducted. One of the leaders in this transformation has been the Rare Tumor Committee of the Gynecologic Oncology Group (GOG), which was established in 2005. In 2014, the GOG merged with other cooperative groups to form the new NRG Oncology cooperative group. Since 2005, several clinical trials for rare ovarian/peritoneal cancer subtypes—clear cell carcinomas, mucinous carcinomas, and low-grade serous carcinomas, and non-epithelial tumors—have been activated.
The overarching principles by which the GOG (NRG) Rare Tumor Committee has operated have included the following: (I) separate clinical trials for distinct histologic subtypes; (II) investigation of novel targeted agents based on promising pre-clinical studies, whenever possible; and (III) inclusion of robust tissue acquisition and translational research components within each trial.
Nevertheless, the study of rare ovarian cancers remains logistically challenging for a variety of reasons. Small patient numbers within each of the subtypes represents a threat to meeting accrual targets. This realization has led to strategies to overcome this limitation, including intergroup trials and international consortia or other collaborations. Additional issues include the implementation of novel trial designs with which to efficiently study these rare tumors and accurate pathologic diagnostic criteria for eligibility. For example, prospective digital pathology review rather than the usual post-hoc central pathology review is necessary for trial screening in most of such investigations. Furthermore, the financial, regulatory, and nursing and data management efforts associated with opening any clinical trials are particularly burdensome when one considers that any single institution may accrue a relatively small number of patients to a multi-institutional or cooperative group trial of a rare tumor.
This review will focus on two rare subtypes of epithelial ovarian cancer—low-grade serous carcinoma and mucinous carcinoma—and will provide an overview of progress to date and research opportunities for the future.
Low-grade serous carcinoma
Background
The story of the evolution of progress in the study of low-grade serous carcinoma of the ovary/peritoneum really began in the early 1990s when Dr. Silva first proposed the binary grading system for serous carcinoma (1). After over a decade of experience using this system rather than that of the International Federation of Gynecology and Obstetrics (FIGO), the findings were reported in 2004 (2). This seemingly trivial proposal for replacement of the time-honored 3-tier grading system (grade 1-3) with the 2-tier system (low grade and high grade) actually galvanized the medical community to seriously study the significant differences between low- and high-grade serous carcinoma in terms of molecular biology and clinical behavior. Prior to this time, FIGO grade 2 serous carcinoma was not well characterized and was considered by some to be more like FIGO grade 1 and by others to be more associated with FIGO grade 3.
Over the ensuing decade since this initial report, the binary grading system for serous carcinoma has been widely studied and ultimately adopted (3-10). During this same period, the molecular biology of low-grade serous carcinoma has begun to be elucidated (11-31), and the clinical behavior better understood (32-46).
Pathology
The binary grading system for serous carcinoma is based primarily on the assessment of nuclear atypia with the mitotic count used as a secondary criterion (2). In their study of 100 cases of serous ovarian carcinoma—50 low-grade and 50 high-grade—from the MD Anderson Cancer Center files, Malpica et al. reported that, in comparison with the FIGO grading system, all but one of the 36 FIGO grade 1 cases were classified as low-grade, and all of the 11 FIGO grade 3 cases were classified as high-grade (2). However, of the 53 FIGO grade 2 cases, 15 were classified as low-grade and 38 as high-grade (2). The results of this study simply underscore the confusion surrounding the FIGO grade 2 category and why migrating to a 2-tier grading system makes so much sense. A further important finding of this study was the coexistence of serous tumor of low malignant potential and low-grade serous carcinoma in 60% of cases. Subsequent reports only further strengthened the observation that the FIGO grading system is flawed and the wisdom surrounding dichotomization of the grading system for serous carcinoma (3,4,6-8). Bodurka et al. conducted an ancillary study of GOG Protocol 158 in which 241 cases of serous carcinoma from the paclitaxel/carboplatin arm of the trial, which had been classified using the FIGO grading system, were re-classified using the MD Anderson binary grading system (8). When analyzed using the original FIGO grading system, there was no difference in clinical outcome in patients with grade 2 or 3 tumors in multivariate analysis (8).
Molecular biology
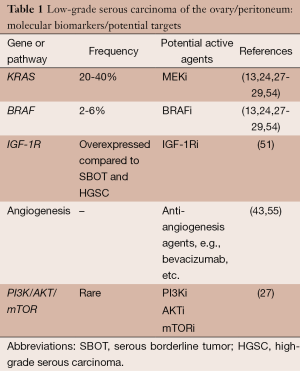
Molecular and genetic investigations over the past decade have brought the biology of low-grade serous carcinoma into much sharper focus. Based on available evidence, we currently believe that low-grade serous carcinoma may arise following an initial diagnosis of serous tumor of low malignant potential or de novo (32-34,47-49). The weight of evidence further suggests that the mitogen-activated protein kinase (MAPK) pathway plays a prominent role in the pathogenesis of both entities. Genomic profiling studies have demonstrated that low-grade serous carcinomas segregate from high-grade serous carcinomas but are similar to serous tumors of low malignant potential (15,17). Compared with high-grade serous carcinomas, low-grade serous carcinomas have a much lower frequency of p53 mutations or p53 expression (18,19), greater expression of estrogen receptor (ER) and progesterone receptor (PR) (21), greater expression of PAX2 (23), overexpression of anterior gradient homolog 3 (AGR3) (50), and overexpression of insulin like growth factor 1 (IGF-1) (51). Although germline BRCA mutations occur in a relatively high proportion of women with high-grade serous carcinoma, low-grade serous carcinoma does not appear to be part of the hereditary breast-ovarian cancer syndrome (52,53).
In 2003, Singer et al. reported their study of 182 ovarian tumors, including 51 serous tumors of low malignant potential and 21 low-grade serous carcinomas (13). KRAS mutations were reported in 33% of serous tumors of low malignant potential and in 35% of low-grade serous carcinomas, and BRAF mutations were found in 28% and 33%, respectively. Subsequent reports of low-grade serous carcinoma, however, seemed to confirm a 20-40% frequency of KRAS mutations but a much lower frequency of BRAF mutations—2-6% (24,54). Based on their findings, Wong et al. concluded that the low frequency of BRAF mutations in advanced stage low-grade serous carcinomas compared with serous tumors of low malignant potential suggested that the former are more likely derived from serous tumors of low malignant potential without BRAF mutations (24). A more recent study appeared to confirm these observations (28). In other words, the presence of a BRAF mutation in an advanced stage serous tumor of low malignant potential may somehow protect against the development of a subsequent low-grade serous carcinoma. In a study of 23 patients with an original diagnosis of serous tumor of low malignant potential who subsequently recurred with low-grade serous carcinoma, patients with KRAS G12V mutations had shorter survival times than those with either KRAS G12D, wild-type, or rare KRAS variants (HR =4.77; P=0.023) (29). And, although it appears that aberrations of the PI3K/AKT/mTOR pathways are relatively rare in low-grade serous carcinoma (27), there is some evidence that dual blockade of the MAP kinase and PI3K/AKT/mTOR pathways may be associated with enhanced activity compared with MAP kinase pathway blockade alone (see below).
Clinical behavior
Surgery is a major modality of treatment in low-grade serous carcinoma, as it is in all histologic subtypes. For most patients, primary surgery, including surgical staging for patients with apparent early-stage disease and cytoreductive surgery for those with metastatic disease, is the initial treatment. Fertility-sparing surgery is an option for selected young patients. For selected women with extensive metastatic disease or significant co-morbidities, neoadjuvant chemotherapy with interval cytoreductive surgery may be recommended. In such cases, either fine needle aspiration/core biopsy or a minimally invasive surgical procedure to establish an accurate diagnosis is performed prior to starting chemotherapy.
Several predominant themes have emerged from studies of the clinical course of low-grade serous carcinoma of the ovary or peritoneum. In an ancillary study of GOG protocol 182, Fader et al. reported the details regarding 189 patients with FIGO grade 1 serous carcinoma (a surrogate for low-grade serous carcinoma) (40). On multivariate analysis, only residual disease status following primary surgery was significantly associated with overall survival (OS). Patients with microscopic residual disease had a significantly longer median progression-free survival (PFS) (33.2 months) and OS (96.9 months) compared with those with residual 0.1-1.0 cm disease (14.7 and 44.5 months, respectively) and more than 1.0 cm of residual disease (14.1 and 42.0 months, respectively). The overall pattern of these results closely resembles that of epithelial ovarian cancer in general. In a second study from the same dataset, serum CA 125 values were analyzed (42). Although pre-treatment CA 125 was not prognostic of outcome, patients with CA 125 levels that normalized after 1-3 cycles of chemotherapy were 60-64% less likely to experience disease progression as compared to those whose CA 125 levels never normalized or normalized after four cycles (P≤0.024). Normalization of CA 125 levels before the second cycle was negatively associated with death, with an HR of 0.45 (P=0.025).
Previs et al. reported the Duke experience with 81 women with low-grade serous carcinoma of the ovary (44). On multivariate analysis, obesity (HR =2.8) and optimal tumor debulking (HR =0.05) were significant predictors of OS. Additionally, obesity was not associated with worse disease-specific survival, suggesting that mortality of obese patients may have been attributable to other comorbidities.
In the initial systematic study of metastatic low-grade serous carcinoma of the ovary by Gershenson et al., in which 112 women with stages II-IV low-grade serous carcinoma were retrospectively analyzed, major features included a relatively young age at diagnosis (median age =43 years), prolonged OS (median OS =82 months) compared with high-grade ovarian cancers, and relative chemoresistance as reflected by the surrogate marker of persistent tumor at the completion of primary treatment (48% of patients) (32). After adjusting for other variables, persistent disease after primary chemotherapy was associated with a shorter PFS time (HR =2.64; P=0.03). The theme of relative chemoresistance, thought to be related to the indolent nature of low-grade serous carcinoma, was subsequently also observed in reports of patients treated with neoadjuvant chemotherapy (34), patients with primary peritoneal low-grade serous carcinoma (36), and patients with recurrent disease (35). Nevertheless, chemotherapy generally remains the standard therapy for women with low-grade serous carcinoma until such time that it is replaced by evidence-based alternative treatment. In addition, in the report of chemotherapy for recurrent low-grade serous carcinoma, 60% of women had stable disease (SD) for a period of time. Whether the frequency of stable disease is more related to tumor biology or a therapeutic effect remains unresolved.
For some women, hormonal therapy may offer a greater benefit than chemotherapy with less associated toxicity (39). In a report of 64 women with recurrent low-grade serous carcinoma who received 89 separate hormonal therapy regimens, 9% of patient-regimens resulted in an objective response, and 62% of patient-regimens resulted in SD (39). In addition, ER/PR expression data were available in 50 patients in this study. Patients with ER+/PR- tumors had a shorter time to progression (HR =1.8) than patients with ER+/PR+ tumors; however, this observation approached but did not reach statistical significance (P=0.056). Thus, hormonal therapy remains a reasonable and potentially active treatment for women with metastatic low-grade serous carcinoma.
Given the realization that cytotoxic chemotherapy has limited activity in low-grade serous carcinoma, a search for more effective systemic therapies is warranted. As with most cancer types, investigators have principally focused on the study of targeted therapies over the past few years. Coupled with these efforts is the continued study of the molecular biology of low-grade serous carcinoma through additional basic science and translational research studies.
Targeted therapeutics
Based on preclinical research findings, potential genes or pathways for targeting low-grade serous carcinoma include the MAPK pathway, IGFR-1, the angiogenesis pathway, and possibly the PI3K/AKT/mTOR pathway (Table 1). The MAPK signaling pathway is one of the most activated and best characterized in cancer (56). The MAPK cascade is triggered by the binding of a ligand that ultimately leads to phosphorylation of ERK (57,58). Thus, MEK is a good candidate for targeted therapy, and a number of MEK inhibitors (MEKi) have been developed in the past few years (59,60). Preclinical studies of ovarian cancer demonstrated significant growth inhibition in cell lines with KRAS or BRAF mutations compared with cell lines with wild-type cells (61,62). In view of the cumulative data indicating mutations within the MAPK pathway, as discussed above, exploration of MEKi in patients with low-grade serous carcinoma was a natural progression.
Full table
In a landmark GOG phase II trial (GOG 0239), Farley et al. demonstrated promising results with a MEKi, selumetinib (54). Fifty two women with recurrent low-grade serous carcinoma were enrolled in this trial and treated with the MEKi, selumetinib 50 mg twice daily. The overall response rate (ORR) was 15%, with one complete response (CR) and seven partial responses (PRs). Another 65% of patients in the trial had SD. The median PFS was 11.0 months. The most common toxicities were gastrointestinal (GI) [13], dermatologic [9], and metabolic [7]. Three patients experienced grade 4 toxicities—one each cardiac, pain, and pulmonary. Mutational analysis was conducted on formalin-fixed, paraffin-embedded (FFPE) tumor samples from 34 patients in this trial. The primary tumor accounted for 82% of the cases. In these 34 cases, there were 2 (6%) BRAF mutations and 14 (41%) KRAS mutations. In this study, there was no correlation between mutations of BRAF or KRAS and objective response. Subsequently, the promising results of this trial in the context of the relatively low response rates of low-grade serous carcinoma to either chemotherapeutic or hormonal agents prompted further investigations.
Three ongoing phase II or III clinical trials have emerged from this experience. Each of these trials includes a different MEKi. The MILO trial (NCT01849874) is an open-label phase III protocol that randomizes patients with recurrent low-grade serous carcinoma to either chemotherapy [physician’s choice of pegylated liposomal doxorubicin (PLD), paclitaxel, or topotecan] or MEK162. A second trial (NCT01936363) has a randomized phase II design and includes the MEKi, pimasertib, with either placebo or SAR245409 (a PI3K/mTOR inhibitor). GOG 0281 is a randomized phase II/III trial (NCT02101788) that has been activated through NRG Oncology. This trial includes a randomization between standard of care (physician’s choice of letrozole, tamoxifen, PLD, weekly paclitaxel, or topotecan) and MEKi monotherapy, trametinib. This trial also includes a robust translational research component, with fresh and archival FFPE tissue for next generation sequencing and proteomics as well as cell-free DNA and pharmacokinetic studies.
As noted above, the angiogenesis pathway may also be a target in patients with low-grade serous carcinoma. Bidus et al. reported three patients with apparent recurrent low-grade serous carcinomas (one with primary peritoneal low-grade serous carcinoma, one with ovarian low-grade serous carcinoma, and another with a mixed low-grade serous-endometrioid carcinoma) treated with bevacizumab, a monoclonal antibody against the vascular endothelial growth factor A (VEGF-A) (55). All three patients experienced a sustained response—two PRs and one CR. Subsequently, Grisham et al. reported on 17 patients with low-grade serous carcinoma of the ovary or peritoneum who received bevacizumab (43). Two patients were treated with single-agent bevacizumab and the others with a combination of bevacizumab and chemotherapy. Fifteen patients were evaluable for response, and six (40%) had a PR. An additional five (33.3%) had SD lasting 3 months or longer.
To date, there have been no clinical trials exploring the role of IGF1-R targeted therapy in women with low-grade serous carcinoma. Likewise, although an agent targeting the PI3K/AKT/mTOR pathway in combination with a MEKi was administered to a proportion of women on one of the three trials above (NCT01936363), the results of this trial are pending, and no AKT inhibitor, PI3K inhibitor, or mTOR inhibitor monotherapy trials specifically for patients with low-grade serous carcinoma have been developed.
Mucinous carcinoma
Background
As with low-grade serous carcinoma, our understanding of the pathologic diagnosis, molecular biology, and clinical behavior of mucinous carcinoma has significantly expanded over the past decade or so. Historically, mucinous carcinoma has been treated similarly to all other epithelial ovarian cancer subtypes. Our new knowledge has sharpened our focus on potential methods of improving treatment for women with this histologic subtype. However, its previously unanticipated extreme rarity has been an impediment to the development of prospective clinical trials.
Pathology
Mucinous tumors appear to represent a spectrum, from benign to borderline to invasive. The pathology of mucinous tumors of the ovary is complex and beyond the scope of this article. However, a few salient points are noted. Ovarian mucinous carcinoma is divided into intraepithelial (non-invasive) carcinoma and invasive carcinoma. Intraepithelial mucinous carcinoma is characterized by the presence of marked atypia but without stromal invasion. Invasive mucinous carcinoma is characterized by stromal invasion more than 5 mm or more than 10 mm (2). Invasive mucinous carcinoma is further divided into two types: (I) expansile (confluent) and (II) infiltrative (63,64). The prognosis of the infiltrative type is significantly worse than that of the expansile type.
The greatest challenge in the pathologic diagnosis of mucinous carcinoma is distinguishing a primary ovarian tumor from a tumor metastatic to ovaries, typically arising in the (GI) tract. Immunohistochemistry may be helpful in distinguishing a primary mucinous carcinoma of the ovary from GI tumors metastatic to ovaries but is somewhat limited. In a study by Vang et al., a CK7+/CK20+ profile was the most common profile in primary ovarian tumors (74%), upper GI tract tumors (78%), and endocervical tumors (88%) but was occasionally observed in lower GI tumors (65). A CK7-/CK20+ profile was the most common profile in lower intestinal tract tumors (79%) and was uncommon in upper GI tract tumors (9%) and rarely observed in primary ovarian tumors (4%). A CK7+/CK20− profile was seen in some primary ovarian tumors (23%), upper GI tract tumors (13%), but not in lower GI tumors. Zaino et al. reviewed 44 cases classified as primary mucinous carcinomas within the context of a large phase III trial (66). The cases were reviewed independently by three pathologists. The pathologists reclassified the majority (57-63%) of mucinous carcinomas as metastatic to the ovary rather than as a primary ovarian tumor.
Molecular biology
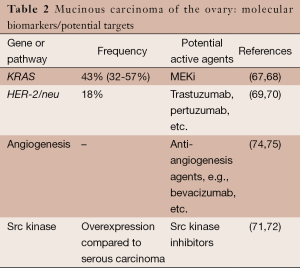
Similar to low-grade serous carcinoma, primary mucinous carcinoma of the ovary has several molecular alterations that may serve as targets for systemic therapy. Approximately 43% (32-56% in various studies) of mucinous carcinomas have a KRAS mutation (67,68).
HER-2/neu amplification has also been observed in mucinous carcinoma of the ovary. McAlpine and colleagues found that 18.2% of 33 mucinous carcinomas contained HER-2/neu amplification (69). In a study of mucinous carcinomas from 49 Asian women, Chao et al. observed an 18.4% frequency of HER-2/neu positivity (70).
Another potential therapeutic target in mucinous carcinoma is src (71,72). And finally, based on experience with treatment of colorectal cancer, the angiogenesis pathway is also a potential target (73). In addition, angiogenesis biomarkers such as microvessel density have been observed to be increased in mucinous ovarian carcinoma compared with other histologic subtypes (74,75).
Clinical behavior
The same surgical principles as outlined above for low-grade serous carcinoma apply to mucinous carcinoma as well. However, based on a retrospective review of 107 patients with mucinous carcinoma of the ovary, Schmeler et al. have made the case that routine lymphadenectomy may not be necessary (76).
For women with stage IA mucinous carcinoma of the ovary, the prognosis is good. There is no definite evidence that adjuvant chemotherapy is beneficial in this subset. Whether patients with stage IC mucinous carcinoma require adjuvant therapy remains controversial. For all patients with stages II-IV mucinous carcinoma, standard therapy has consisted of paclitaxel/carboplatin ×6 cycles, or a variation on this theme (intraperitoneal chemotherapy, dose-dense paclitaxel regimen, etc.). Over the past decade, however, it has become increasingly clear that advanced stage or recurrent mucinous carcinoma has a significantly worse prognosis compared with serous carcinoma (77-83).
The Hellenic Cooperative Oncology Group reported their experience with 141 patients with stage III and IV epithelial ovarian cancer treated with primary platinum-based chemotherapy (77). The outcomes of 47 patients with mucinous carcinoma were compared with those of 97 serous carcinoma patients. The ORR was 38.5% in patients with mucinous carcinoma and 70% in patients with serous carcinoma. However, there were no significant differences in time to progression or OS. Hess et al. reported their experience with 81 women with advanced epithelial ovarian cancer who underwent primary platinum-based chemotherapy (78). Comparing the outcomes of 27 patients with mucinous carcinoma with 54 patients with other histologic subtypes, they found that that the mucinous carcinoma patients had significantly inferior response rate (26.3% vs. 64.9%), median PFS rate (5.7 vs. 14.1 months), and median OS rate (12.0 vs. 36.7 months). Three additional pooled analyses have documented significantly worse outcomes in women with mucinous carcinoma compared with those with serous carcinoma (79-81). In a study of The Surveillance, Epidemiology, and End Results (SEER) database, Schiavone reported on 40,571 women treated between 1988 and 2007 for epithelial ovarian cancer (82). The database included 4,811 patients with mucinous carcinoma; those with advanced stage disease had inferior cancer-specific survival compared with patients with serous carcinoma.
Experience with patients with recurrent mucinous carcinoma of the ovary also indicates a worse outcome compared with other histologic subtypes. Pignata reported 20 patients with recurrent mucinous carcinoma and 388 patients with recurrent cancer of other histologic subtypes—all with platinum-sensitive disease treated with platinum-based chemotherapy (83). The response rate for the mucinous carcinoma patients was significantly worse—36.4% vs. 62.6% (P=0.04). Thus, novel therapies for women with mucinous carcinoma of the ovary are clearly needed.
Targeted therapeutics
Based on preclinical research findings, potential genes or pathways for targeting mucinous carcinoma include HER-2/neu amplification, KRAS, src, and the angiogenesis pathway (Table 2). However, there is very limited information on experience with targeted therapy in women with mucinous carcinoma of the ovary.
Full table
McAlpine et al. reported one patient with recurrent mucinous carcinoma of the ovary who experienced a dramatic response to the anti HER2 monoclonal antibody, trastuzumab, in combination with various chemotherapeutic agents (69). Related to the high frequency of KRAS mutations in colorectal cancer, targeting of the MAP kinase pathway has not produced promising results to date (31,59). For example, Hochster et al. conducted a phase II study of selumetinib plus irinotecan as second-line therapy in patients with KRAS-mutated colorectal cancer (84). Three patients (9.7%) had a PR, and 16 patients had stable disease for ≥4 weeks. To date, there have been no trials of a MEKi in patients with recurrent mucinous carcinoma of the ovary. Likewise, there have not yet been any trials of src inhibitors in this patient population.
The mEOC/GOG 0241 trial was designed to study the activity of a colorectal cancer-type regimen in women with newly diagnosed metastatic mucinous carcinoma of the ovary. This was a phase III trial that randomized women to either the control arm of paclitaxel/carboplatin ×6 cycles vs. the combination of capecitabine/oxaliplatin ×6 cycles. Additionally, there was a secondary randomization to bevacizumab or no bevacizumab to test the activity of anti-angiogenesis therapy. The trial was opened in both the UK and US but suffered from slow accrual related to the rarity of ovarian mucinous carcinoma and was closed prematurely.
Summary
In summary, while our understanding of low-grade serous carcinoma of the ovary/peritoneum and mucinous carcinoma of the ovary has expanded significantly over the past decade, the experience with targeted therapy for these rare histologic subtypes is still quite limited. However, the good news is that our approach to the treatment of ovarian/peritoneal/fallopian tube cancer has been transformed during the same period. No longer are we continuing to pursue the “one size fits all” strategy. Currently, for low-grade serous carcinoma, the emphasis is on defining the activity of MEKi therapy, or, in some cases, combinations of MEKi and inhibitors of the PI3K/AKT/mTOR pathway. On the other hand, the extreme rarity of mucinous carcinoma of the ovary combined with the failure of mEOC/GOG 0241 has, for the time being, put a damper on separate targeted agent trials for women with this subtype. The hope is that lessons learned from this experience will inform the design and conduct of future trials. In summary, there is tremendous potential for progress against these two rare histologic subtypes by leveraging our knowledge of their molecular biology and translating this understanding into improved, novel therapeutics.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Franco Muggia and Eleonora Teplinsky) for the series “Epithelial Ovarian Cancer Treatment: Integrating Molecular Targeting” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2015.01.05). The series “Epithelial Ovarian Cancer Treatment: Integrating Molecular Targeting” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Silva EG, Gershenson DM. Standardized histologic grading of epithelial ovarian cancer: elusive after all these years. Gynecol Oncol 1998;70:1. [PubMed]
- Malpica A, Deavers MT, Lu K, et al. Grading ovarian serous carcinoma using a two-tier system. Am J Surg Pathol 2004;28:496-504. [PubMed]
- Seidman JD, Horkayne-Szakaly I, Cosin JA, et al. Testing of two binary grading systems for FIGO stage III serous carcinoma of the ovary and peritoneum. Gynecol Oncol 2006;103:703-8. [PubMed]
- Malpica A, Deavers MT, Tornos C, et al. Interobserver and intraobserver variability of a two-tier system for grading ovarian serous carcinoma. Am J Surg Pathol 2007;31:1168-74. [PubMed]
- Roth LM. Two-tier grading system for ovarian epithelial cancer: has its time arrived? Am J Surg Pathol 2007;31:1285-7. [PubMed]
- Vang R, Shih IeM, Salani R, et al. Subdividing ovarian and peritoneal serous carcinoma into moderately differentiated and poorly differentiated does not have biologic validity based on molecular genetic and in vitro drug resistance data. Am J Surg Pathol 2008;32:1667-74. [PubMed]
- Vang R, Shih IeM, Kurman RJ. Ovarian low-grade and high-grade serous carcinoma: pathogenesis, clinicopathologic and molecular biologic features, and diagnostic problems. Adv Anat Pathol 2009;16:267-82. [PubMed]
- Bodurka DC, Deavers MT, Tian C, et al. Reclassification of serous ovarian carcinoma by a 2-tier system: a Gynecologic Oncology Group Study. Cancer 2012;118:3087-94. [PubMed]
- Bell DA. Low-grade serous tumors of ovary. Int J Gynecol Pathol 2014;33:348-56. [PubMed]
- Kurman RJ, Carcangiu ML, Herrington CS, et al. eds. WHO Classification of Tumours of Female Reproductive Organs. Lyon: International Agency for Research on Cancer (IARC), 2013.
- Singer G, Kurman RJ, Chang HW, et al. Diverse tumorigenic pathways in ovarian serous carcinoma. Am J Pathol 2002;160:1223-8. [PubMed]
- Singer G, Shih IeM, Truskinovsky AM, et al. Mutational Analysis of K-ras Segregates Ovarian Serous Carcinomas into Two Types: Invasive MPSC (Low-grade Tumor) and Conventional Serous Carcinoma (High-grade Tumor). International Journal of Gynecological Pathology 2003;22:37-41. [PubMed]
- Singer G, Oldt R 3rd, Cohen Y, et al. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J Natl Cancer Inst 2003;95:484-6. [PubMed]
- Gilks CB. Subclassification of ovarian surface epithelial tumors based on correlation of histologic and molecular pathologic data. Int J Gynecol Pathol 2004;23:200-5. [PubMed]
- Meinhold-Heerlein I, Bauerschlag D, Hilpert F, et al. Molecular and prognostic distinction between serous ovarian carcinomas of varying grade and malignant potential. Oncogene 2005;24:1053-65. [PubMed]
- Sieben NL, Macropoulos P, Roemen GM, et al. In ovarian neoplasms, BRAF, but not KRAS, mutations are restricted to low-grade serous tumours. J Pathol 2004;202:336-40. [PubMed]
- Bonome T, Lee JY, Park DC, et al. Expression profiling of serous low malignant potential, low-grade, and high-grade tumors of the ovary. Cancer Res 2005;65:10602-12. [PubMed]
- O'Neill CJ, Deavers MT, Malpica A, et al. An immunohistochemical comparison between low-grade and high-grade ovarian serous carcinomas: significantly higher expression of p53, MIB1, BCL2, HER-2/neu, and C-KIT in high-grade neoplasms. Am J Surg Pathol 2005;29:1034-41. [PubMed]
- Singer G, Stöhr R, Cope L, et al. Patterns of p53 mutations separate ovarian serous borderline tumors and low- and high-grade carcinomas and provide support for a new model of ovarian carcinogenesis: a mutational analysis with immunohistochemical correlation. Am J Surg Pathol 2005;29:218-24. [PubMed]
- Szymanska-Pasternak J, Szymanska A, Medrek K, et al. CHEK2 variants predispose to benign, borderline and low-grade invasive ovarian tumors. Gynecol Oncol 2006;102:429-31. [PubMed]
- Wong KK, Lu KH, Malpica A, et al. Significantly greater expression of ER, PR, and ECAD in advanced-stage low-grade ovarian serous carcinoma as revealed by immunohistochemical analysis. Int J Gynecol Pathol 2007;26:404-9. [PubMed]
- Kuo KT, Guan B, Feng Y, et al. Analysis of DNA copy number alterations in ovarian serous tumors identifies new molecular genetic changes in low-grade and high-grade carcinomas. Cancer Res 2009;69:4036-42. [PubMed]
- Tung CS, Mok SC, Tsang YT, et al. PAX2 expression in low malignant potential ovarian tumors and low-grade ovarian serous carcinomas. Mod Pathol 2009;22:1243-50. [PubMed]
- Wong KK, Tsang YT, Deavers MT, et al. BRAF mutation is rare in advanced-stage low-grade ovarian serous carcinomas. Am J Pathol 2010;177:1611-7. [PubMed]
- May T, Virtanen C, Sharma M, et al. Low malignant potential tumors with micropapillary features are molecularly similar to low-grade serous carcinoma of the ovary. Gynecol Oncol 2010;117:9-17. [PubMed]
- Shih IeM, Kurman RJ. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol 2004;164:1511-8. [PubMed]
- Jones S, Wang TL, Kurman RJ, et al. Low-grade serous carcinomas of the ovary contain very few point mutations. J Pathol 2012;226:413-20. [PubMed]
- Grisham RN, Iyer G, Garg K, et al. BRAF mutation is associated with early stage disease and improved outcome in patients with low-grade serous ovarian cancer. Cancer 2013;119:548-54. [PubMed]
- Tsang YT, Deavers MT, Sun CC, et al. KRAS (but not BRAF) mutations in ovarian serous borderline tumour are associated with recurrent low-grade serous carcinoma. J Pathol 2013;231:449-56. [PubMed]
- Escobar J, Klimowicz AC, Dean M, et al. Quantification of ER/PR expression in ovarian low-grade serous carcinoma. Gynecol Oncol 2013;128:371-6. [PubMed]
- Burotto M, Chiou VL, Lee JM, et al. The MAPK pathway across different malignancies: a new perspective. Cancer 2014;120:3446-56. [PubMed]
- Gershenson DM, Sun CC, Lu KH, et al. Clinical behavior of stage II-IV low-grade serous carcinoma of the ovary. Obstet Gynecol 2006;108:361-8. [PubMed]
- Shvartsman HS, Sun CC, Bodurka DC, et al. Comparison of the clinical behavior of newly diagnosed stages II-IV low-grade serous carcinoma of the ovary with that of serous ovarian tumors of low malignant potential that recur as low-grade serous carcinoma. Gynecol Oncol 2007;105:625-9. [PubMed]
- Schmeler KM, Sun CC, Bodurka DC, et al. Neoadjuvant chemotherapy for low-grade serous carcinoma of the ovary or peritoneum. Gynecologic Oncology 2008;108:510-4. [PubMed]
- Gershenson DM, Sun CC, Bodurka D, et al. Recurrent low-grade serous ovarian carcinoma is relatively chemoresistant. Gynecol Oncol 2009;114:48-52. [PubMed]
- Schmeler KM, Sun CC, Malpica A, et al. Low-grade serous primary peritoneal carcinoma. Gynecol Oncol 2011;121:482-6. [PubMed]
- Schlumbrecht MP, Sun CC, Wong KN, et al. Clinicodemographic factors influencing outcomes in patients with low-grade serous ovarian carcinoma. Cancer 2011;117:3741-9. [PubMed]
- Diaz-Padilla I, Malpica AL, Minig L, et al. Ovarian low-grade serous carcinoma: a comprehensive update. Gynecol Oncol 2012;126:279-85. [PubMed]
- Gershenson DM, Sun CC, Iyer RB, et al. Hormonal therapy for recurrent low-grade serous carcinoma of the ovary or peritoneum. Gynecol Oncol 2012;125:661-6. [PubMed]
- Fader AN, Java J, Ueda S, et al. Survival in women with grade 1 serous ovarian carcinoma. Obstet Gynecol 2013;122:225-32. [PubMed]
- Romero I, Sun CC, Wong KK, et al. Low-grade serous carcinoma: new concepts and emerging therapies. Gynecol Oncol 2013;130:660-6. [PubMed]
- Fader AN, Java J, Krivak TC, et al. The prognostic significance of pre- and post-treatment CA-125 in grade 1 serous ovarian carcinoma: a gynecologic Oncology Group study. Gynecol Oncol 2014;132:560-5. [PubMed]
- Grisham RN, Iyer G, Sala E, et al. Bevacizumab shows activity in patients with low-grade serous ovarian and primary peritoneal cancer. Int J Gynecol Cancer 2014;24:1010-4. [PubMed]
- Previs RA, Kilgore J, Craven R, et al. Obesity is associated with worse overall survival in women with low-grade papillary serous epithelial ovarian cancer. Int J Gynecol Cancer 2014;24:670-5. [PubMed]
- Takeuchi S, Lucchini M, Schmeler KM, et al. Utility of 18F-FDG PET/CT in follow-up of patients with low-grade serous carcinoma of the ovary. Gynecol Oncol 2014;133:100-4. [PubMed]
- Crane EK, Sun CC, Ramirez PT, et al. The role of secondary cytoreduction in low-grade serous ovarian cancer or peritoneal cancer. Gynecol Oncol 2015;136:25-9. [PubMed]
- Crispens MA, Bodurka DC, Deavers MT, et al. Response and Survival in Patients With Progressive or Recurrent Serous Ovarian Tumors of Low Malignant Potential. Obstetrics & Gynecology 2002;99:3-10. [PubMed]
- Gershenson DM, Silva EG, Tortolero-Luna G, et al. Serous borderline tumors of the ovary with noninvasive peritoneal implants. Cancer 1998;83:2157-63. [PubMed]
- Gershenson DM, Silva EG, Levy L, et al. Ovarian serous borderline tumors with invasive peritoneal implants. Cancer 1998;82:1096-103. [PubMed]
- King ER, Tung CS, Tsang YT, et al. The anterior gradient homolog 3 (AGR3) gene is associated with differentiation and survival in ovarian cancer. Am J Surg Pathol 2011;35:904-12. [PubMed]
- King ER, Zu Z, Tsang YT, et al. The insulin-like growth factor 1 pathway is a potential therapeutic target for low-grade serous ovarian carcinoma. Gynecol Oncol 2011;123:13-8. [PubMed]
- Press JZ, De Luca A, Boyd N, et al. Ovarian carcinomas with genetic and epigenetic BRCA1 loss have distinct molecular abnormalities. BMC Cancer 2008;8:17. [PubMed]
- Vineyard MA, Daniels MS, Urbauer DL, et al. Is low-grade serous ovarian cancer part of the tumor spectrum of hereditary breast and ovarian cancer? Gynecol Oncol 2011;120:229-32. [PubMed]
- Farley J, Brady WE, Vathipadiekal V, et al. Selumetinib in women with recurrent low-grade serous carcinoma of the ovary or peritoneum: an open-label, single-arm, phase 2 study. Lancet Oncol 2013;14:134-40. [PubMed]
- Bidus MA, Webb JC, Seidman JD, et al. Sustained response to bevacizumab in refractory well-differentiated ovarian neoplasms. Gynecol Oncol 2006;102:5-7. [PubMed]
- McCubrey JA, Steelman LS, Chappell WH, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta 2007;1773:1263-84.
- Friday BB, Adjei AA. Advances in targeting the Ras/Raf/MEK/Erk mitogen-activated protein kinase cascade with MEK inhibitors for cancer therapy. Clin Cancer Res 2008;14:342-6. [PubMed]
- Jing J, Greshock J, Holbrook JD, et al. Comprehensive predictive biomarker analysis for MEK inhibitor GSK1120212. Mol Cancer Ther 2012;11:720-9. [PubMed]
- Neuzillet C, Tijeras-Raballand A, de Mestier L, et al. MEK in cancer and cancer therapy. Pharmacol Ther 2014;141:160-71. [PubMed]
- Miller CR, Oliver KE, Farley JH, et al. MEK1/2 inhibitors in the treatment of gynecologic malignancies. Gynecol Oncol 2014;133:128-37. [PubMed]
- Pohl G, Ho CL, Kurman RJ, et al. Inactivation of the mitogen-activated protein kinase pathway as a potential target-based therapy in ovarian serous tumors with KRAS or BRAF mutations. Cancer Res 2005;65:1994-2000. [PubMed]
- Nakayama N, Nakayama K, Yeasmin S, et al. KRAS or BRAF mutation status is a useful predictor of sensitivity to MEK inhibition in ovarian cancer. Br J Cancer 2008;99:2020-8. [PubMed]
- Lee KR, Scully RE. Mucinous tumors of the ovary: a clinicopathologic study of 196 borderline tumors (of intestinal type) and carcinomas, including an evaluation of 11 cases with ‘pseudomyxoma peritonei’. Am J Surg Pathol 2000;24:1447-64. [PubMed]
- Rodríguez IM, Prat J. Mucinous tumors of the ovary: a clinicopathologic analysis of 75 borderline tumors (of intestinal type) and carcinomas. Am J Surg Pathol 2002;26:139-52. [PubMed]
- Vang R, Gown AM, Barry TS, et al. Cytokeratins 7 and 20 in primary and secondary mucinous tumors of the ovary: analysis of coordinate immunohistochemical expression profiles and staining distribution in 179 cases. Am J Surg Pathol 2006;30:1130-9. [PubMed]
- Zaino RJ, Brady MF, Lele SM, et al. Advanced stage mucinous adenocarcinoma of the ovary is both rare and highly lethal: a Gynecologic Oncology Group study. Cancer 2011;117:554-62. [PubMed]
- Kelemen LE, Köbel M. Mucinous carcinomas of the ovary and colorectum: different organ, same dilemma. Lancet Oncol 2011;12:1071-80. [PubMed]
- Rechsteiner M, Zimmermann AK, Wild PJ, et al. TP53 mutations are common in all subtypes of epithelial ovarian cancer and occur concomitantly with KRAS mutations in the mucinous type. Exp Mol Pathol 2013;95:235-41. [PubMed]
- McAlpine JN, Wiegand KC, Vang R, et al. HER2 overexpression and amplification is present in a subset of ovarian mucinous carcinomas and can be targeted with trastuzumab therapy. BMC Cancer 2009;9:433. [PubMed]
- Chao WR, Lee MY, Lin WL, et al. Assessing the HER2 status in mucinous epithelial ovarian cancer on the basis of the 2013 ASCO/CAP guideline update. Am J Surg Pathol 2014;38:1227-34. [PubMed]
- Matsuo K, Nishimura M, Bottsford-Miller JN, et al. Targeting SRC in mucinous ovarian carcinoma. Clin Cancer Res 2011;17:5367-78. [PubMed]
- Kim HS, Han HD, Armaiz-Pena GN, et al. Functional roles of Src and Fgr in ovarian carcinoma. Clin Cancer Res 2011;17:1713-21. [PubMed]
- Recondo G Jr, Díaz-Cantón E, de la Vega M, et al. Advances and new perspectives in the treatment of metastatic colon cancer. World J Gastrointest Oncol 2014;6:211-24. [PubMed]
- Nakanishi Y, Kodama J, Yoshinouchi M, et al. The expression of vascular endothelial growth factor and transforming growth factor-beta associates with angiogenesis in epithelial ovarian cancer. Int J Gynecol Pathol 1997;16:256-62. [PubMed]
- Orre M, Lotfi-Miri M, Mamers P, et al. Increased microvessel density in mucinous compared with malignant serous and benign tumours of the ovary. Br J Cancer 1998;77:2204-9. [PubMed]
- Schmeler KM, Tao X, Frumovitz M, et al. Prevalence of lymph node metastasis in primary mucinous carcinoma of the ovary. Obstet Gynecol 2010;116:269-73. [PubMed]
- Pectasides D, Fountzilas G, Aravantinos G, et al. Advanced stage mucinous epithelial ovarian cancer: the Hellenic Cooperative Oncology Group experience. Gynecol Oncol 2005;97:436-41. [PubMed]
- Hess V, A'Hern R, Nasiri N, et al. Mucinous epithelial ovarian cancer: a separate entity requiring specific treatment. J Clin Oncol 2004;22:1040-4. [PubMed]
- Winter WE 3rd, Maxwell GL, Tian C, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol 2007;25:3621-7. [PubMed]
- Mackay HJ, Brady MF, Oza AM, et al. Prognostic relevance of uncommon ovarian histology in women with stage III/IV epithelial ovarian cancer. Int J Gynecol Cancer 2010;20:945-52. [PubMed]
- Alexandre J, Ray-Coquard I, Selle F, et al. Mucinous advanced epithelial ovarian carcinoma: clinical presentation and sensitivity to platinum-paclitaxel-based chemotherapy, the GINECO experience. Ann Oncol 2010;21:2377-81. [PubMed]
- Schiavone MB, Herzog TJ, Lewin SN, et al. Natural history and outcome of mucinous carcinoma of the ovary. Am J Obstet Gynecol 2011;205:480.e1-8.
- Pignata S, Ferrandina G, Scarfone G, et al. Activity of chemotherapy in mucinous ovarian cancer with a recurrence free interval of more than 6 months: results from the SOCRATES retrospective study. BMC Cancer 2008;8:252. [PubMed]
- Hochster HS, Uboha N, Messersmith W, et al. Phase II study of selumetinib (AZD6244, ARRY-142886) plus irinotecan as second-line therapy in patients with K-RAS mutated colorectal cancer. Cancer Chemother Pharmacol 2015;75:17-23. [PubMed]


