Ultrasound-guided secondary radiofrequency ablation combined with chemotherapy in gastric cancer with recurrent liver metastasis
Introduction
Gastric cancer is a kind of common clinical malignant tumors, of which the incidence and mortality accounts for the fourth and second place respectively in the global malignancy. According to the statistics, there are more than 1 million new cases of gastric cancer and about 800,000 deaths worldwide every year (1). In China, its morbidity and mortality rates are ranked second and third in malignant tumors, respectively (2). Because of the atypical clinical symptoms of gastric cancer, most gastric cancer patients have been in the advanced stage when diagnosed. In addition, due to the specificity of anatomical structure and venous return, about 5–14% of patients may have liver metastases (3,4), which is an important cause of death in gastric cancer patients (5), and directly affects patient’s survival and quality of life. Most patients with gastric cancer and liver metastases have lost surgical opportunities, so they are often given palliative care or chemotherapy and radiofrequency ablation (RFA) to prolong the survival period and improve life quality (6-8). In particular, RFA can directly act on the lesions and ablate the lesions. Being safe, effective, and well-tolerated (8), it is a commonly used as alternative surgical method for tumor ablation. A previous study reported that combining RFA with chemotherapy for the treatment of liver metastases from breast cancer could achieve similar survival rate to that of surgery (9). And repeated RFA is the first choice for the treatment of tumor recurrence in situ (10). However, whether the secondary RFA treatment of patients with gastric cancer and liver metastases recurrence after RFA can prolong the survival of patients and improve the quality of life is worth to further explore. In this study, 87 patients with gastric cancer and recurrent liver metastases were selected to investigate the effect of secondary RFA combined with chemotherapy on gastric cancer with recurrent hepatic metastases, thereby providing clinical evidence for this therapy.
Methods
Subjects
A total of 87 patients with gastric cancer and recurrent liver metastases from June 2012 to February 2018 were retrospective analyzed. All patients enrolled met the following inclusion criteria: (I) the primary tumor was pathologically confirmed as gastric cancer; (II) the first RFA confirmed liver metastasis, and the lesion was completely ablated after treatment; (III) the presence of recurrent liver lesions was identified by contrast-enhanced ultrasound, enhanced CT and MR; (IV) patients who signed informed consent. Exclusion criteria: (I) patients with extrahepatic metastases and recurrences; (II) patients with cardiopulmonary insufficiency or who cannot tolerate RFA; (III) patients with chemotherapy contraindications; (IV) those who did not sign informed consent; (V) patients with mental disorders who cannot collaborate (11). According to the treatment approaches, 46 cases were assigned into study group and 41 cases into control group. Our study was in accordance with the Declaration of Helsinki, and was approved by the Medical Ethics Committee of Ningbo No. 2 Hospital (Approval No: PJ-KY-NBEY-2013-001-01).
Methods in detail
Patients’ clinical database, such as general data, treat methods and clinical efficacy, were retrospective analyzed. The study group was treated with RFA plus chemotherapy, while the control group was treated with chemotherapy alone. In the study group, all patients received same RFA treatment, and all received 3 cycles of chemotherapy for 3 weeks before RFA and another 3 cycles of chemotherapy for 3 weeks after RFA. The duration of chemotherapy was 6 months (12).
Chemotherapy regimens: 23 patients in the study group received the FOLFOX6 protocol: Oxaliplatin [Sanofi (Hangzhou) Pharmaceutical Co., Ltd.] 85 mg/m2, intravenous drip infusion for 2 h, day 1; Leucovorin (Hospira Australia Pty Ltd.) 400 mg/m2, intravenous drip infusion for 2 h, day 1; 5-fluorouracil (Shanghai Xudong Haipu Pharmaceutical Co., Ltd.) 2,400 mg/m2, intravenous injection of loading dose, followed by 5 fluorouracil 2,400 mg/m2, continuous intravenous drip infusion for 46 hours.
Thirteen patients received the regimen of teggio + cisplatin: Teggio (Qilu Pharmaceutical Co., Ltd.) 80 mg/m2, intravenous drip infusion, day 1–4; Cisplatin (Qilu Pharmaceutical Co., Ltd.) 75 mg/m2, intravenous drip infusion, day 1–3.
Ten patients received the regimen of irinotecan + 5 fluorouracil: irinotecan [Pfizer (Perth) Pty Limited] 100 mg/m2, intravenous drip infusion, day 1; leucovorin 200 mg/m2, intravenous drip infusion, day 1–2; 5-fluorouracil (Shanghai Xudong Haipu Pharmaceutical Co., Ltd.) 400 mg/m2, intravenous loading dose; 5-fluorouracil 600 mg/m2, continuous intravenous drip infusion for 22 h.
RFA treatment: as shown in Figure 1, Valleylab Coop-tip RF system was used for this treatment. The ultrasound machine was a Siemens Acuson Sequoia 512 ultrasound system. According to the specific position and the tumor size, the thermal coagulation treatment regimen was determined. Then the electroacupuncture was inserted into the center of the lesion under ultrasound guidance. The frequency of the radiofrequency treatment instrument was set at 460 kHz and with the energy of 200 W. The ablation was performed at a thermal coagulation range of 2–5 cm to ensure that the coagulation range is 0.5–1 cm larger than the tumor edge, without damaging other organs. Treatment was evaluated 6 months later.
The control group received chemotherapy only, of which 18 cases received FOLFOX6; 14 received teggio + cisplatin; 9 received irinotecan + 5 fluorouracil. The specific scheme was the same as the study group.
Evaluation criteria
Principal observations indicators: (I) reduced size of lesions: calculated using the criteria proposed by the 1981 World Health Organization. The lesion area is the product of the maximum vertical two-path diameter of the tumor, and the proportion of the reduced size of lesions = (pre-treatment lesion area—after-treatment lesion area)/pre-treatment lesion area; (II) CT scans were performed in both groups after operation and each chemotherapy to evaluate the damage of metastatic lesions, and observe residual lesions or occurrence of new lesions. The median survival time and the survival rates of 6 months, 1 year, and 2 years of the two groups were calculated during follow-up; (III) the relationship between the prognosis of patients and factors including sex, age, treatment approach (chemotherapy alone, RFA plus chemotherapy), synchronous/metachronous liver metastasis, diameter of liver metastases (if it was ≥5 cm), pathological type and number of lesions was analyzed to determine the factors affecting the prognosis.
Secondary observations indicators: (I) baseline data: age, sex, pathological type, and number of lesions; (II) methods for life quality scoring: “0” indicated that the patient completely loses self-care ability; “1” indicated that the patient could not walk independently, but could eat and excrete independently; “2” indicated that the patient could walk with the help of others, and could independently eat and excrete; “3” meant that the patient could completely take care of himself; (III) complications.
Statistical analysis
The data obtained in this study were analyzed using SPSS 19.0 statistical software. Measured data were expressed as mean ± standard deviation (). The comparison between the two groups adopted two-independent samples t-test, and the comparison before and after intervention in the same group adopted paired t-test. Counting data were expressed as rate and analyzed by χ2 test. Rank sum test was used to compare ranked data. The skewed data were represented by the median, and the rank sum test of independent samples was sued for the pairwise comparison. The Kaplan-Meier method was used for survival analysis. Cox regression model was used for the analysis of factors that affecting prognosis. P<0.05 indicates statistical significance.
Results
Comparison of baseline data
As can be seen from Table 1, there was no statistically significant difference between the baseline data of the two groups (all P>0.05).
Table 1
| Group | n | Gender (male/female) | Age (years old) | Pathological type | Number of focus | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Poorly differentiated | Moderately differentiated | Well differentiated | 1 | 2 | ≥3 | |||||
| Study | 46 | 29/17 | 46–67 (60.8±3.2) | 24 | 18 | 4 | 26 | 15 | 5 | |
| Control | 41 | 26/15 | 48–69 (62.4±5.7) | 22 | 14 | 5 | 23 | 12 | 6 | |
| χ2/t | 0.001 | −1.637* | 0.412 | 0.182 | ||||||
| P | 0.971 | 0.105 | 0.814 | 0.856 | ||||||
*, is the value of t, and the rest is the value of χ2.
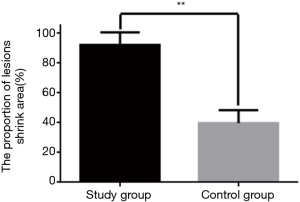
Comparison of the proportion of reduced area in both groups
As shown in Figures 2,3, the proportion of reduced lesions in the study group was significantly higher than that in the control group (P<0.01).
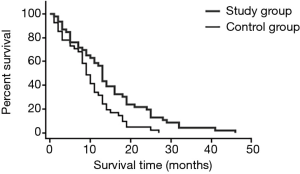
Comparison of survival rates in the two groups
As shown in Table 2, there was no significant difference in half-year survival rates between the two groups (P>0.05). The 1- and 2-year survival rates of the study group were 58.70% and 23.91%, respectively, which were significantly higher than 34.15% and 4.88% of the control group (both P<0.05). See Table 2, Figure 4.
Table 2
| Group | n | Half-year survival rates | 1-year survival rates | 2-year survival rates |
|---|---|---|---|---|
| Study | 46 | 35 (76.09) | 27 (58.70) | 11 (23.91) |
| Control | 41 | 30 (73.17) | 14 (34.15) | 2 (4.88) |
| χ2 value | 0.098 | 5.243 | 6.180 | |
| P value | 0.755 | 0.022 | 0.013 |
Comparison of quality of life in two groups
All patients were followed up for 3 to 46 months, with a median follow-up of 24.9 months. The median survival time of the study group was 26.1 months (95% CI: 10.9, 37.5), which was significantly longer than the 10.4 months of the control group (95% CI: 4.3, 17.3, χ2=288.561, P=0.000). As shown in Table 3, the quality of life for 6 months and 1 year in the study group was significantly higher than those in the control group (both P<0.05).
Table 3
| Group | Half-year quality of life | 1-year quality of life | 2-year quality of life | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scores | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 |
| Study | 2 | 6 | 19 | 8 | 3 | 12 | 9 | 3 | 5 | 4 | 2 | 0 |
| Control | 3 | 12 | 11 | 4 | 5 | 8 | 1 | 0 | 1 | 1 | 0 | 0 |
| Z value | −2.048 | 2.655 | 0.322 | |||||||||
| P value | 0.041 | 0.013 | 0.748 | |||||||||
Complications
No severe complications such as biliary obstruction, intestinal fistula, intraperitoneal hemorrhage, parietal abscess, and pneumothorax were observed in the study group. Four patients experienced mild microwave ablation-related adverse reactions, including abdominal pain (2 cases) and nausea (2 cases). These adverse reactions were relieved by symptomatic supportive treatment.
Factors affecting prognosis
Cox regression model showed that treatment approach, synchronous/metachronous liver metastasis, diameter of liver metastases, pathological stage and number of lesions were influence factors of prognosis (all P<0.05). See Table 4. Furthermore, Cox regression model also showed that treatment approach, diameter of liver metastases, pathological stage and number of lesions were independent influence factors of prognosis. Patients treated with RFA plus chemotherapy, with diameter of liver metastases <5 cm, single lesion, and pathological stage of high differentiation had better prognosis. See Table 5.
Table 4
| Prognostic factor | OR | 95% CI | P value |
|---|---|---|---|
| Age (≤60 vs. >60 years old) | 1.03 | 0.69–1.46 | 0.609 |
| Sex (male vs. female) | 0.95 | 0.66–1.27 | 0.714 |
| Treatment approach (chemotherapy alone, RFA plus chemotherapy) | 2.11 | 1.32–3.35 | 0.002 |
| Diameter of liver metastases (5 vs. <5 cm) | 3.12 | 2.08–4.45 | 0.001 |
| Number of lesions (1 vs. >1) | 2.19 | 1.53–3.09 | 0.001 |
| Pathological stage (poorly vs. moderate to high differentiated) | 1.69 | 1.13–2.32 | 0.023 |
RFA, radiofrequency ablation.
Table 5
| Prognostic factor | OR | 95% CI | P value |
|---|---|---|---|
| Treatment approach (chemotherapy alone, RFA plus chemotherapy) | 1.64 | 1.12–2.65 | 0.042 |
| Diameter of liver metastases (5 vs. <5 cm) | 3.20 | 2.24–4.85 | 0.001 |
| Number of lesions (1 vs. >1) | 1.71 | 1.03–2.55 | 0.016 |
| Pathological stage (poorly vs. moderate to high differentiated) | 1.59 | 1.01–2.63 | 0.009 |
RFA, radiofrequency ablation.
Discussion
Liver is a common organ metastasis site of malignant tumors. Liver metastasis seriously affects the survival time and quality of life of patients. Patients with gastric cancer and recurrent liver metastases are often in the late stage of the disease and lose the chance of surgery (13). Therefore, the treatment purpose of these patients is mainly to improve the quality of life and extend survival time. RFA is an effective method for the treatment of hepatic metastases. It can directly act on tumor lesions, and its coagulation range is 0.5–1.0 cm beyond the edge of the lesion. Besides, it can inactivate the lesions to the maximum extent, and has the advantages of less trauma and can be performed multiple times. Study of Hwang et al. showed that percutaneous ultrasound-guided RFA plus chemotherapy was safe and effective in the treatment of patients with liver metastasis. The overall survival rates of patients in 1, 2, 3 and 5 years was 70.45%, 42.90%, 20.32% and 10.16%, respectively. They also found that chemotherapy after RFA was an important factor affecting the survival of patients, which further confirming the effectiveness of RFA in the treatment of liver metastasis (14). However, due to the high recurrence rate of liver metastasis, there are few reports on the clinical effectiveness of secondary RFA treatment for patients had recurrent liver metastasis. There were still some studies have shown that patients with hepatocellular carcinoma can improve their immune function even if they undergo secondary RFA therapy (15), and they also have good curative effects in liver metastases of malignant tumors (16,17). Therefore, it is feasible to some extent to perform secondary RFA in patients with gastric cancer and recurrent liver metastases. However, RFA treatment also has some deficiencies, that is, it is difficult to remove the tiny lesions. If not removed in time, it will easily lead to recurrence (18,19). Therefore, RFA treatment combined with chemotherapy can kill and inhibit tumor cells to the maximum extent, eliminate small lesions and reduce recurrence (20,21). A number of studies have shown that, the treatment of ultrasound-mediated secondary RFA combined with chemotherapy for a variety of malignant tumors complicated by recurrent liver metastases is satisfactory, and is able to improve the life quality of patients (22,23).
This study showed that for patients with gastric cancer and recurrent liver metastases, the area of focus decreased more significantly in the treatment of secondary RFA combined with chemotherapy compared with single chemotherapy, indicating that RFA can better reduce the tumor load. At the same time, chemotherapy can kill tumor cells in small lesions, the combination of the two can achieve better killing and inhibition of tumor lesions, its efficacy is better than chemotherapy alone. The survival rate and quality of life of the two groups were further compared. The results showed that the 1- and 2-year survival rates of the study group were 58.70% and 23.91%, respectively, which were significantly higher than the 34.15% and 4.88% of the control group. The median survival time of the study group was 26.1 months, significantly longer than the 10.4 months in the control group, suggesting that the second RFA combined with chemotherapy can significantly improve the survival rate of patients with gastric cancer and recurrent liver metastases, effectively prolong survival time, improve the clinical prognosis of gastric cancer and recurrent liver metastases. The quality of life scores in the study group in 6 months and 1 year were significantly higher than the control group (both P<0.05). This shows that the combination of RFA and chemotherapy can better improve the quality of life of patients. The reason might be that RFA combined with chemotherapy can better reduce the number of tumor cells, and may improve the patient’s immune function and further activate the body’s immune response to exhibit its anti-tumor effects (15). During the treatment of the study group, no serious complications such as intrahepatic abscess occurred. The RFA related adverse reactions with digestive system symptoms were all relieved after symptomatic supportive therapy and were able to tolerate treatment. However, due to the small sample size in this study, it is still necessary to confirm this by further expanding the sample and carrying out multi-center studies. At the same time, there was no comparison between the efficacy of different chemotherapy regimens combined with ultrasound-guided RFA, and the next step in the study will focus on research.
This study also analyzed the prognostic factors of gastric cancer with liver metastasis. Results of Cox regression model showed that treatment approach, diameter of liver metastases, pathological stage and number of lesions were independent influence factors of prognosis. Patients treated with RFA plus chemotherapy, with diameter of liver metastases <5 cm, single lesion, and pathological stage of highly differentiation had better prognosis, which indicated that patients with diameter of liver metastases <5 cm, single lesion, and pathological stage of highly differentiation could receive secondary RFA plus chemotherapy for maximum survival.
Conclusions
Ultrasound-mediated secondary RFA combined with chemotherapy in the treatment of gastric cancer and recurrent liver metastases clinical comprehensive efficacy than simple chemotherapy, can effectively improve the quality of life and survival of patients.
Acknowledgments
Funding: This work was supported by
Footnote
Conflict of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.03.45). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was approved by the Medical Ethics Committee of Ningbo No. 2 Hospital and was conducted in accordance with the Helsinki Declaration of 1964 (revised 2008). Due to the retrospective nature of this study, the need for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Timmermans C, Doffagne E, Venet D, et al. Statistical monitoring of data quality and consistency in the Stomach Cancer Adjuvant Multi-institutional Trial Group Trial. Gastric Cancer 2016;19:24-30. [Crossref] [PubMed]
- Huang XY, Han RQ, Teng ZM, et al. Incidence, mortality and survival in rural areas of stomach cancer during 2003-2012 in Jiangsu Province, China. Chinese Journal of Disease Control & Prevention 2017.
- Zhang Y, Zhang Q, Zhang M, et al. DC - SIGNR by influencing the lncRNA HNRNPKP2 upregulates the expression of CXCR4 in gastric cancer liver metastasis. Mol Cancer 2017;16:78. [Crossref] [PubMed]
- Zhang H, Deng T, Liu R, et al. Exosome-delivered EGFR regulates liver microenvironment to promote gastric cancer liver metastasis. Nat Commun 2017;8:15016. [Crossref] [PubMed]
- Wang J, Tong XQ, Lu TS, et al. Transcatheter arterial chemoembolization combined with radiofrequency ablation for the treatment of malignant tumors in specific sites in the liver. Chin J Minim Inva Surg 2016;9-12.
- Liu K, Xia JD, Jin CL, et al. The value of radiofrequency ablation in the treatment of metastatic liver cancer. Journal of Hebei Medicine 2016;22:1674-6.
- Li MX, Jin ZX, Zhou JG, et al. Prognostic Value of Lymph Node Ratio in Patients Receiving Combined Surgical Resection for Gastric Cancer Liver Metastasis: Results from Two National Centers in China. Medicine (Baltimore) 2016;95:e3395. [Crossref] [PubMed]
- Wang H, Liu B, Long H, et al. Clinical study of radiofrequency ablation combined with TACE in the treatment of breast cancer with liver metastasis. Oncol Lett 2017;14:2699-702. [Crossref] [PubMed]
- Carrafiello G, Fontana F, Cotta E, et al. Ultrasound-guided thermal radiofrequency ablation (RFA) as an adjunct to systemic chemotherapy for breast cancer liver metastases. Radiol Med 2011;116:1059-66. [Crossref] [PubMed]
- Xie X, Jiang C, Peng Z, et al. Local Recurrence after Radiofrequency Ablation of Hepatocellular Carcinoma: Treatment Choice and Outcome. J Gastrointest Surg 2015;19:1466-75. [Crossref] [PubMed]
- Lee JW, Choi MH, Lee YJ, et al. Radiofrequency ablation for liver metastases in patients with gastric cancer as an alternative to hepatic resection. BMC Cancer 2017;17:185. [Crossref] [PubMed]
- Yang CX, Cheng W, Yuan SS, et al. The efficacy of ultrasound mediated radiofrequency ablation combined with chemotherapy in the treatment of recurrent hepatic metastases. Journal of Modern Oncology 2016;24:1092-1095.
- Nielsen K, Scheffer HJ, Volders JH, et al. Radiofrequency Ablation to Improve Survival After Conversion Chemotherapy for Colorectal Liver Metastases. World J Surg 2016;40:1951-8. [Crossref] [PubMed]
- Hwang JE, Kim SH, Jin J, et al. Combination of percutaneous radiofrequency ablation and systemic chemotherapy are effective treatment modalities for metachronous liver metastases from gastric cancer. Clin Exp Metastasis 2014;31:25-32. [Crossref] [PubMed]
- Huang H, Zhao XW, Xie B. Efficacy and influence of ultrasound-guided radiofrequency ablation on the immunological function and liver function of patients with hepatocellular carcinoma. Journal of Clinical and Experimental Medicine 2017;16:892-5.
- Xu J, Zhao Y. Clinical efficacy of radiofrequency ablation combined with chemotherapy in breast cancer patients with liver metastases. Journal of Medical Research 2017;46:59-62.
- Li YB, Cao MM, Hu XG, et al. Radiofrequency ablation combined with transcatheter arterial chemoembolization and cetuximab in the treatment of liver metastases from colorectal cancer. Journal of Interventional Radiology 2016;25:129-33.
- Yoon IS, Shin JH, Han K, et al. Ultrasound-Guided Intraoperative Radiofrequency Ablation and Surgical Resection for Liver Metastasis from Malignant Gastrointestinal Stromal Tumors. Korean J Radiol 2018;19:54-62. [Crossref] [PubMed]
- Huang T, Zhong JH, Qi YP, et al. Clinical efficacy of radiofrequency ablation for postoperative recurrent and primary hepatocellular carcinoma. Chinese Journal of General Surgery 2019;34:936-9.
- Mao R, Zhao JJ, Zhao H, et al. Non-response to preoperative chemotherapy is a contraindication to hepatectomy plus radiofrequency ablation in patients with colorectal liver metastases. Oncotarget 2017;8:75151-61. [Crossref] [PubMed]
- Li W, Cai GY, Wu M, et al. Radiofrequency ablation combined with systemic chemotherapy in the management of nasopharyngeal carcinoma with liver metastasis. Zhonghua Yi Xue Za Zhi 2016;96:2629. [PubMed]
- Ding XY, Chen JL, Sun W, et al. Influence of time schedule of radiofrequency ablation on the safety and prognosis of patients with colorectal cancer liv-er metastasis receiving XELOX regimen: A single-center clinical study. Journal of Clinical Hepatology 2018;34:2603-9.
- Wang Z, Pan H, Fang Y. Retrospective study of radiofrequency ablation for colorectal carcinoma combined with liver metastasis. Anhui Medical Journal 2016;12:1491-4.