
Targeting angiogenesis: vascular endothelial growth factor and related signaling pathways
Epithelial ovarian cancer (EOC) is the 7th most common cancer among women, with an estimated 225,000 new cases and 140,000 deaths worldwide in 2014 (1). The high mortality of this cancer is related to the lack of symptoms until advanced stage disease is present (2). Treatment of EOC consists of a surgical cytoreduction to reduce the burden of disease, followed by adjuvant chemotherapy. Platinum and taxane combinations have been the standard of care for first line treatment of EOC since the 1990s. Since introduction of these agents, overall survival (OS) curves have remained relatively unchanged. While 80% of patients achieve remission with first line treatment, the majority of patients will relapse and ultimately die of their disease (3). Resistance to platinum chemotherapy is generally acquired by multiple mechanisms including increased repair of sub-lethal DNA damage and inhibition of apoptosis. Platinum-refractory and platinum-resistant tumors show modest clinical response or significant survival gains with traditional treatments.
Based on this resistance and toxicities of chemotherapy, new avenues in EOC treatment include targeting specific tumor mutations and directing treatment at the tumor microenvironment. The tumor microenvironment includes dysfunctional molecular pathways including angiogenesis or neovascularization.
Angiogenesis
EOC spreads through the peritoneal cavity when floating malignant cells survive and proliferate in other areas of the abdomen. The survival of the tumors in part requires new blood vessel development or angiogenesis. Tumors without neovascularization are unable to grow larger than 1-2 mm3 (4). Normal tissues and organs rely on a balance of angiogenic and anti-angiogenic forces to control growth and development. The process of angiogenesis becomes unbalanced in favor of new vessel formation in malignancy. Tumor neovascularization therefore plays a pivotal role in the survival and dissemination of EOC.
The theory of angiogenesis, which stated that specific factors could stimulate new vasculature, first appeared in a publication in 1939 by Ide and Warren (5). In 1971, Dr. Folkman hypothesized that tumor growth is dependent on the angiogenic pathway and that inhibition of angiogenesis could be used to treat cancer (4,6).
Multiple pathways exist in the angiogenic cascade. The vascular endothelial growth factor (VEGF) pathway is the most widely studied. VEGF was first isolated and cloned by Genentech, Inc. The group demonstrated that mice without VEGF had a dramatically reduced ability to form tumors (7). VEGF binds to a tyrosine kinase surface receptor that spans the cell membrane. Activation occurs thru transphosphorylation. The most studied VEGF receptors are VEGFR-1, VEGFR-2, and VEGFR-3. VEGF is up regulated in EOC, thereby promoting angiogenesis, and cellular adhesion. Elevated levels of VEGF are found in ascites. In a review of 529 patients from six studies, high serum levels of VEGF correlate with a higher risk of death and recurrence and may be an independent prognostic factor for OS (8).
Since tumor growth is reliant on neovascularization, novel molecular agents try to block the VEGF pathway by binding to the ligand (e.g., bevacizumab, aflibercept), the extracellular portion of the receptor (e.g., ramucirumab) or by inhibiting the tyrosine kinase effects on the intracellular portion of the receptor (e.g., pazopanib, cediranib, nintedanib).
Genentech developed the first of these agents, bevacizumab, which is a humanized monoclonal antibody against VEGF (9-11). When bevacizumab binds to VEGF, it blocks endothelial activation and prevents new blood vessels from forming, which are necessary for continued tumor growth. In tumors, the neovascularization is disorganized and the vascular architecture is defective or leaky. The leaky vessels allow for increased vascular permeability, hypoxia, and high interstitial fluid. In addition to preventing new vessels from forming, bevacizumab leads to vascular normalization. The normal architecture is restored in the remaining vessels in the tumor, which is theorized to improve blood supply to the tumor, which increases exposure to oxygen, nutrients and chemotherapy drug delivery. The improved treatment response with the addition of bevacizumab to chemotherapy may be related to this increased exposure to chemotherapy. Tumor normalization could also negatively impact the effect of molecular agents by preventing penetration of the antibody into the tumor (12-14). Bevacizumab has been widely studied in many tumor sites and it is the most studied angiogenesis inhibitor in EOC. Another VEGF inhibitor is aflibercept. This agent is a fusion protein consisting of the binding domains of VEGFR-1 and VEGFR-2 and functions as a high-affinity decoy receptor of all VEGF ligands and placental like growth factor (PlGF) (15,16).
Angiogenesis is also mediated thru VEGF-independent pathways including fibroblast growth factors (FGF), platelet-derived growth factor (PDGF), and angiopoietins. FGF binds to the FGF receptors 1 and 2 and activates angiogenesis. PDGF recruits pericytes and assists with blood vessel maturation (17). Cediranib is an oral inhibitor of VEGFR [1,2,3], PDGR alpha and beta, FGRF [1], and c-kit. Nintedanib is a potent inhibitor of VEGFR [1,2,3], PDGFR (alpha, beta), and FGFR [1,2,3], members of the v-src sarcoma viral oncogene homolog family, and fms-like tyrosine kinase 3. Pazopanib, which is currently FDA approved for sarcomas and renal cell carcinoma, is an oral multiple tyrosine kinase inhibitor including VEGFR [1, 2, 3], c-KIT, FGFR, and PDGFR (alpha, beta). Sorafenib targets VEGFR [2,3] and PDGFR-beta as well as c-kit, FLT-3, and v-raf 1 murine leukemia viral oncogene humalog. Vandetanib is an oral inhibitor of VEGFR-, EGFR- and RET-signaling. The angiopoietin family binds to the tyrosine kinase receptor, Tie2, which causes vascular remolding by promoting endothelial sprouting and by stabilizing endothelial junctions (18). Trebananib (formerly AMG 386) is a peptide-Fc fusion protein that prevents the activation of the Tie2 receptor by angiopoietin 1 and 2. The VEGF-independent pathways may allow for resistance to agents acting only on VEGF inhibition (e.g., bevacizumab, aflibercept). Anti-angiogenesis agents that inhibit multiple pathways may overcome this resistance (e.g., pazopanib, cediranib, nintedanib, sorafenib, vandetanib). The VEGF-dependent and VEGF independent pathways cause a cascade of downstream events including activation of the PI3K-Akt-mTOR and Ras-Raf-MEK-Erk pathways. Drugs in development on these downstream targets include the Ras-Raf-ERK pathway (e.g., enzastaurin, dabrafenib, vemurafenib, etc.) and the mTOR pathways (e.g., temsirolimus, sirolimus, everolimus, rapamycin, etc.).
Further downstream from the VEGF pathways, the Notch pathway has also been implicated in the tumorigenesis of serous ovarian carcinomas. Four notch receptors (Notch 1, 2, 3, and 4) are known to have five corresponding receptors [jagged 1, jagged 2, delta like family (Dll) 1, 3, 4]. VEGF signaling activates the Notch pathway to increase Notch ligands, Dll4 (19). This up regulation of Notch ligands subsequently down regulates VEGF receptors (20). Inhibition of Notch/Dll4 can up regulate VEGF receptors (21). The Notch pathways function on the cell cycle depends on the cellular environment (22). When comparing gene arrays between tumor containing ovarian epithelial cells and benign ovarian cells, jagged 1 and Dll4 were noted to be upregulated (23,24). Increased levels of Dll4 were noted to have comparatively poor survival overall when compared to low Dll4 expression (25). When Dll4 was silenced in mouse models (26), decreased tumor growth and decreased angiogenesis were noted, an effect that was made even more pronounced by the addition of bevacizumab. Given the prevalence and cross talk in this pathway, several targets are available. Notch signaling starts with receptor-ligand binding, which results in a conformational change that allows gamma secretase, an intracytoplasmic enzyme, access to its substrate. By inhibiting gamma secretase, downstream activation of Notch target genes is prevented (27). Gamma secretase inhibitors (GSI) are the most commonly studied. While it is unknown if GSIs will play a treatment role in ovarian, fallopian tube or peritoneal cancers, several GSIs are undergoing phase I/II studies in EOC.
Vascular disrupting agents (VDAs) are a large group of compounds that specifically target endothelial cells through disruption of the cytoskeleton and cell-to-cell junctions, inducing morphological changes and apoptosis in endothelial cells. This leads to a cascade of events ultimately reducing vascular flow, stasis and occlusion. The inhibition of blood flow and the consequent hypoxia induces necrosis to the core of tumor cells growing in proximity of mature vasculature (28,29). Pre-clinically, this effect has been observed in the center of the tumor, which becomes necrotic, with a rim of highly proliferative and VEGF-sensitive cells at the periphery. This rim of tumor cells probably survives because it derives nutritional support from nearby normal blood vessels, which are less responsive to VDAs but are the main targets of the anti-angiogenic agents, supporting the development of combination strategies.
Phase II/III trials have studied novel anti-angiogenics in primary and recurrent EOC. Clinical trials have demonstrated benefit in response rate (RR) and progression free survival (PFS) with anti-angiogenic agents. Approval by the United States Food and Drug Administration (FDA) in the past has required improvement in OS. A measurable benefit in OS however has not been achieved with anti-angiogenic agents in EOC. The lack of benefit in EOC is likely related to the high crossover rates among treatment and the inability to control for treatments patients receive after the trial closes. Based on phase III results, bevacizumab currently has the European Commission (EU) approval for treatment of primary and recurrent EOC. In the fall of 2014, the FDA approved bevacizumab for recurrent platinum resistant EOC. To date no other anti-angiogenics have been FDA approved for use in ovarian cancer.
Methods
We designed a systematic literature review to identify published prospective phase II/III clinical trials of anti-angiogenic agents in women with histologically proven EOC, fallopian tube cancer (FTC) or primary peritoneal cancer (PPC). PubMed/Medline databases were searched from 1 January 2002 to 1 December 2014, using the terms: AEE788; aflibercept; AMG 386; angiogenesis inhibitors; anti-VEGF; bevacizumab; BIBF 1120; cediranib; fosbretabulin; imatinib; nintedanib; pazopanib; perifosine; saracatinib; sorafenib; sunitinib; trebananib; vandetanib; VEGF; VEGF-receptor AND ovarian cancer OR FTC OR primary peritoneal cancer. Congress abstracts from American Society of Clinical Oncology (ASCO), Society of Gynecologic Oncology (SGO), and European Society of Gynaecologic Oncology (ESGO) were also searched for these agents. Results were limited to peer-reviewed, English language articles only. Reviews, meta-analyses, case reports, editorials, and letters were excluded.
Direct VEGF inhibitors
Bevacizumab
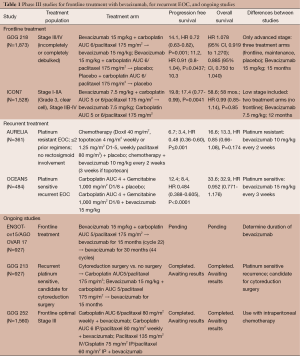
The first anti-angiogenesis agent to progress to Phase III trials in EOC was bevacizumab. Four phase III trials in EOC have been published, two with frontline and two with recurrent disease indications (Table 1). The two frontline trials, GOG 218 and ICON7, were published in the same issue of The New England Journal of Medicine in 2011. In 2005, the Gynecologic Oncology Group (GOG) initiated GOG 218 as a double-blind placebo controlled study that examined the role of bevacizumab in frontline treatment. A total of 1,873 patients with previously untreated Stage III and IV EOC from 336 sites and four countries were enrolled. Patients were stratified based on performance status, stage and debulking status. Optimal cytoreduction (less than 1 cm of disease) after surgery was achieved in 34% of patients. Forty percent of patients had suboptimal debulking with residual tumor greater than 1 cm, and the remaining 26% of patients had Stage IV disease. Stage III patients were not eligible if they had no gross residual disease after cytoreductive surgery. Originally, the protocol allowed only Stage III suboptimal disease and all Stage IV patients but was amended to allow optimally cytoreduced Stage III patients who still had gross residual disease following surgery. Patients were randomized in a 1:1:1 fashion to three treatment arms: standard treatment (carboplatin plus paclitaxel every 21 days) with placebo, standard treatment with bevacizumab (15 mg/kg every 21 days), and standard treatment with bevacizumab followed by maintenance bevacizumab for up to 10 months (16 cycles). The primary endpoint was PFS, which was also a protocol amendment during the study. The original primary endpoint had been OS. Progression was defined by a rise in CA 125 level, by Response Evaluation Criteria in Solid Tumors (RECIST) criteria, global deterioration of health, or death. Compared to standard therapy, the PFS for the bevacizumab maintenance treatment group was 3.8 months longer than standard therapy (14.1 vs. 10.3 months; HR 0.72; 95% CI, 0.63-0.82; P=0.001). When compared to standard therapy with bevacizumab, the addition of maintenance therapy showed a 2.9-month increase in PFS (14.1 vs. 11.2 months). In a secondary analysis, CA 125 was removed from PFS analysis, and the time to progression was 12 months in the standard treatment group and 18 months in the maintenance group. No difference in OS was seen between the three treatment arms. On a secondary analysis, an increase in OS was seen in Stage IV patients treated with bevacizumab maintenance compared to standard therapy [40.6 vs. 32.8 months, HR 0.72 (0.53-0.97)] (30). The most common adverse event (AE) was hypertension (7.2% with standard therapy vs. 16.5% with bevacizumab during chemotherapy only vs. 22.9% for the bevacizumab maintenance cohort).
Full table
In Europe, the International Collaborative Ovarian Neoplasm (ICON) 7 trial (31) was a two armed, an open label trial that compared standard chemotherapy (carboplatin and paclitaxel every 3 weeks for six cycles) to standard therapy plus bevacizumab (7.5 mg/kg every 3 weeks for five or six cycles and continued for 12 additional cycles or until disease progression). The study enrolled 1,528 patients with high risk Stage I and Stage II, III, and IV EOC. Similar to the results of GOG 218, the primary endpoint, PFS, was improved by approximately 2 months (19.0 vs. 17.3 months; HR 0.85; 95% CI, 0.70-0.94; P=0.004) in patients receiving bevacizumab over standard therapy. When the high-risk group (patients with suboptimally debulked Stage IIIC and Stage IV tumors) was evaluated separately, the estimated median improvement in PFS was increased to 5.4 months (10.5 months with standard therapy vs. 15.9 months with bevacizumab, HR 0.68; 95% CI, 0.55 to 0.85; P<0.001). OS was a secondary endpoint and again was not different between the two groups (58.6 vs. 58.0 months respectively, HR 0.99, P=0.85). However, OS did show an improvement of 9.4 months (30.3 vs. 39.7 months; P=0.0072, HR 0.64; 95% CI, 0.48-0.85; P=0.002) in patients with high-risk disease treated with bevacizumab compared to standard therapy (32). The AEs in the bevacizumab group were similar to the results in GOG 218.
GOG 218 and ICON7 both showed an improvement in PFS for EOC with upfront treatment with bevacizumab, but the improvement of PFS was less in ICON7. The difference in outcome may be related to the differences between the studies. The study protocols differed with ICON7 having two treatment arms while GOG 218 included a third arm with standard treatment including bevacizumab without maintenance therapy. The dose of bevacizumab used in the studies was different; ICON7 used a lower dose of 7.5 mg/kg compared to 15 mg/kg every 21 days used in GOG 218. Maintenance treatments were allowed for 16 cycles (11 months) in GOG 218 compared to 12 cycles (8 months) in ICON7. The differences in administration of bevacizumab between GOG 218 and ICON7 raised the question of the ideal dose and duration of bevacizumab. AGO-OVAR17 or the BOOST (Bevacizumab Ovarian Optimal Standard Treatment) Trial is an ongoing study looking at the optimal treatment duration of maintenance bevacizumab in frontline treatment. Patients receive chemotherapy (carboplatin/paclitaxel) with bevacizumab followed by either 16 or 38 cycles of maintenance therapy. The primary endpoint is PFS. The study has reached 50% enrollment, and results are expected in 2017.
Given the improvement in PFS with the use of bevacizumab with three week dosing of carboplatin and paclitaxel, the question was raised asking whether bevacizumab is additive to other frontline regimens. GOG 252, which closed to enrollment in 2011, is a randomized 3-arm study in optimally cytoreduced EOC patients evaluating the use of bevacizumab and intraperitoneal chemotherapy. Patients are randomized to arm I (weekly paclitaxel, carboplatin and bevacizumab 15 mg/kg every 3 weeks), arm II (weekly paclitaxel, intraperitoneal carboplatin every 3 weeks, and bevacizumab), or arm III (paclitaxel and bevacizumab every 3 weeks with intraperitoneal cisplatin and paclitaxel). ANTHALYA is an ongoing phase II study looking at bevacizumab, carboplatin, and paclitaxel for neoadjuvant therapy (33). No results are available at this time. OCTAVIA (34-36), a phase II single arm study, evaluated bevacizumab with weekly paclitaxel and carboplatin every three weeks followed by bevacizumab maintenance therapy. PFS, the primary endpoint, was 24.1 months (95% CI, 19.9-33.3 months; PFS events in 56% of patients). One- and 2-year OS rates were 97.8% (95% CI, 95.7-99.9%) and 92.3% (95% CI, 88.4-96.2%), respectively. GOG 262 evaluated dose-dense (weekly paclitaxel) vs. conventional paclitaxel (37). The phase III study allowed bevacizumab at the physician’s discretion. 80% of patients were placed on bevacizumab. No difference in PFS was seen between the dose-dense and the conventional treatment (HR 0.97, 95% CI, 0.79-1.18). When the patients were subdivided based on bevacizumab usage, patients not receiving bevacizumab were noted to have an improvement in PFS (median, 14 vs. 10 months, HR 0.60, 95% CI, 0.37-0.96) similar to that seen in Japanese Gynecologic Oncology Group 3016 study. The lack of improvement of PFS with bevacizumab continues to raise the question of the optimal administration of anti-angiogenesis agents.
The phase III studies of bevacizumab in recurrent EOC are the OCEANS, AURELIA, and GOG 213 trials. The OCEANS study was a randomized, double blind trial of 484 patients with platinum sensitive recurrent disease. Inclusion criteria included measurable disease, no prior treatments for recurrent disease, and no prior VEGF/VEGFR treatments. Patients were randomized to chemotherapy (carboplatin/gemcitabine) with placebo followed by placebo maintenance vs. chemotherapy with bevacizumab followed by bevacizumab maintenance therapy until disease progression. PFS, the primary endpoint, was improved by 4 months in patients treated with bevacizumab (12.4 vs. 8.4 months; HR 0.484; 95% CI, 0.388-0.605; P≤0.0001). Similar to frontline treatment, no difference was seen in OS (35.2 vs. 33.3 months). However, 31% of patients in the control group were treated with bevacizumab; the high rate of crossover of bevacizumab makes the impact of maintenance therapy on OS difficult to interrupt (38).
While the OCEANS study focused on platinum sensitive EOC, the AURELIA study looked at patients with platinum-resistant recurrent EOC. Patients needed to progress within 6 months of receiving four or more cycles of platinum based chemotherapy and have a histologically proven recurrence. Patients could not have had greater than two prior regimens; prior anti-angiogenesis therapy, however, was not an exclusion criterion. A total of 331 patients were randomized to physician’s choice chemotherapy [pegylated liposomal doxorubicin (PLD), topotecan, or paclitaxel] alone or in combination with bevacizumab until disease progression. Given historical low RRs in this group of EOC, no maintenance arm was included. A 3.3-month improvement in PFS, the primary endpoint, was found in the combination treatment group (6.7 vs. 3.4 months; HR 0.48; 95% CI, 0.38-0.60; P≤0.001). The combination treatment group had a higher RR (27.3% vs. 11.8%; P=0.001). As in the prior studies, no difference was seen in OS. However, a high cross over rate was noted with 40% of patients in the chemotherapy treatment arm receiving bevacizumab after progression (39). In a subgroup analysis of the AURELIA trial, OS was evaluated by chemotherapy type. In the patients treated with PLD, median OS was 13.7 months in the bevacizumab plus PLD group vs. 14.1 months (HR 0.92; 95% CI, 0.62-1.36) in the PLD only group. For the topotecan-treated group, median OS was 13.8 vs. 13.3 months (HR 1.07; 95% CI, 0.72-1.67), respectively. However, in patients treated with weekly paclitaxel, median OS was 22.4 vs. 13.2 months (HR 0.65; 95% CI, 0.42-1.02), respectively. A 35% relative improvement in OS was noted in the weekly paclitaxel and bevacizumab treatment group (40). While the results are promising, the AURELIA trial was not powered for this subgroup analysis and these results will need to be evaluated in a prospective study.
GOG 213 is an ongoing phase III randomized control trial looking at platinum sensitive recurrent EOC and secondary debulking. Candidates for secondary cytoreduction were randomized to receive secondary cytoreduction surgery or no surgery followed by randomization to standard chemotherapy (carboplatin/paclitaxel) with or without bevacizumab. The study met the accrual goal in 2011 for evaluating chemotherapy and the remaining enrolled patients could select their chemotherapy (carboplatin/paclitaxel or carboplatin/gemcitabine with or without bevacizumab). Results are expected in the spring of 2015.
Based on the improvement of PFS seen in platinum sensitive and recurrent EOC, numerous phase II studies have looked at bevacizumab in combination with other approved treatment agents for recurrent EOC. Phase II studies have been conducted with cyclophosphamide (41-43), doxil (44-46), gemcitabine and carboplatin (47,48), oxaliplatin and docetaxel (49), irinotecan (50), topotecan (51), albumin bound paclitaxel (52), and gemcitabine and oxaliplatin (53). Other phase II studies have looked at bevacizumab alone (54-56) and in combination with other anti-angiogenesis agents including sorafenib (57), erlotinib (58,59), and fosbretabulin tromethamine. Additional phase II studies have examined use with a folate antimetabolite, pemetrexed (60), and mTOR inhibitor, everolimus (61), and with an inhibitor of tubulin, eribulin and oxaliplatin (62). The results are mixed with varied RRs and toxicities.
Aflibercept
Phase II studies with aflibercept have shown mixed results. Aflibercept’s effect on the timing of paracentesis for symptom control has been evaluated in two phase II studies. Patients with recurrent EOC with symptomatic ascites requiring three or more paracenteses at a frequency of 1-4 paracenteses per month were given aflibercept 4 mg/kg every 2 weeks. A response was seen in 62.5% (95% CI, 35.4-84.8%) of patients with a median time to paracentesis of 76 days (95% CI, 64.0-178) compared to 16.8 days (63). In another trial (64), a significant increase in median time to paracentesis was seen in the treatment group of 55.1 vs. 23.3 days. In a phase II study, patients were randomized to aflibercept at a dose of either 2 or 4 mg/kg every 2 weeks until they developed disease progression or significant toxicity. The primary endpoint of complete response (CR) or partial response (PR) by RECIST criteria was not met in either arm (65). However, aflibercept at a dose of 6 mg/kg combined with 75 mg/m2 docetaxel every three weeks demonstrated a 54% RR by RECIST criteria with 11 CRs and 14 PRs (66).
VEGF independent inhibitors
Cediranib
Phase II studies evaluated the tyrosine kinase inhibitor, cediranib, (30 mg daily) in recurrent EOC. One study reported a 30% clinical benefit rate (CBR) with 17% PRs (67). A second trial (68) divided the patients by platinum sensitivity and showed a RR of 41% in platinum sensitive patients and 29% in platinum resistant patients. A randomized, double blind phase III study ICON6 (69,70) evaluated cediranib in recurrent platinum sensitive EOC in 456 patients. The study had three arms: platinum based chemotherapy (carboplatin AUC 5/6 and paclitaxel 175 mg/m2), platinum based therapy with cediranib, and the combination therapy followed by cediranib maintenance therapy. The primary endpoints were PFS and OS. PFS was 9.4 months with platinum chemotherapy alone vs. 11.4 months (HR 0.67; 95% CI, 0.53-0.87; log-rank test P=0.0003) with chemotherapy and cediranib and 12.6 months (HR 0.57; 95% CI, 0.45-0.74; log-rank test P=0.00001) with maintenance therapy. OS increased by 2.7 months in the maintenance group from 17.6 to 20.3 (HR, 0.70; log-rank test P=0.042).
Cediranib has also been evaluated in combination with the PARP inhibitor, olaparib. In this phase II study, recurrent platinum sensitive EOC or BRCA related EOC with measurable disease and no prior anti-angiogenesis/PARP agents were randomized to cediranib 30 mg daily in combination with oral olaparib 400 mg BID or olaparib alone. The RR was 84% in the patients treated with combination therapy compared to 56% in patients only treated with olaparib (HR 2.9, 95% CI, 1.5-5.6, P=0.001). However, the overall rate of grade 3 and 4 AEs was higher for combination patients (70%) than with olaparib alone (7%): fatigue (27% vs. 7%), diarrhea (23% vs. 0%), and hypertension (39% vs. 0%), respectively (71).
Nintedanib
In a phase II trial, patients with recurrent EOC were randomized to nintedanib 250 mg twice daily or placebo for maintenance therapy following a response to their last chemotherapy. At the endpoint of 36 weeks, PFS was 16.3% with nintedanib vs. 5% (HR 0.65; 95% CI, 0.42-1.02; P=0.06) with placebo (72). Incidence of AEs was similar in both arms. Based on positive phase II results, the Eastern Cooperative Oncology Group (ECOG) initiated a phase III study for frontline treatment of EOC (AGO-OVAR12/LUME-Ovar1) to investigate nintedanib with carboplatin/paclitaxel followed by maintenance nintedanib therapy vs. carboplatin/paclitaxel and placebo. Treatment was for a maximum of 120 weeks. The primary endpoint was PFS. Results were presented at ESGO (October 2013) (73); 1,366 patients demonstrated minimal difference in median PFS with the addition of nintedanib vs. the control arm (17.3 vs. 16.6 months, HR 0.84; 95% CI, 0.72-0.98; P=0.0239). A subgroup analysis showed that nintedanib might be most effective in patients with <1 cm of disease compared to patients with high tumor residual (median PFS of 27.1 vs. 20.8 months; HR 0.75; 95% CI, 0.61-0.92; P=0.005). The OS data remains immature, and final results have not been published at this time.
Pazopanib
Combining pazopanib with traditional therapy appears non-feasible based on results of a phase I/II trial of pazopanib in combination with standard chemotherapy (carboplatin and paclitaxel) (74). The study was terminated after 33% of patients experienced a dose limiting toxicity including two gastrointestinal bowel perforations and 50% of patients had severe myelotoxicity. A phase II, open label study of single agent pazopanib was conducted in patients with recurrent EOC that had a complete CA 125 response to therapy (75). Pazopanib 800 mg daily resulted in an overall RR of 18%, and a 31% CA125 response.
Based on the tolerability and promising activity as a single agent, the AGO study group (AGO-OVAR16) completed an international randomized double blind phase III trial. Nine hundred and forty patients were randomized to pazopanib 800 mg daily or placebo as a maintenance therapy after traditional chemotherapy for up to 24 months (76). Patients receiving pazopanib had a prolonged PFS by RECIST criteria with 17.9 months compared to 12.3 months (HR =0.766; 95% CI, 0.64-0.91; P=0.0021). OS results are pending. While pazopanib significantly increased PFS in women receiving it as primary maintenance therapy, a significant toxicity was noted with 33.3% vs. 5.6% discontinuing treatment based on AE. Grade 3 or 4 AE included hypertension, neutropenia, liver related toxicity and fatigue (77).
The role of pazopanib was also explored in recurrent EOC. MITO-11 is a recently presented open-label, randomized phase II trial of weekly paclitaxel plus/minus pazopanib in platinum resistant EOC. MITO-11 demonstrated significant improvement in PFS (median 6.3 vs. 3.5 months, HR 0.42; 95% CI, 0.25-0.69) as well as RR (50% vs. 21%; P=0.03). Median OS was 14.8 months (95% CI, 9.1-NA) with paclitaxel and 18.7 months (95% CI, 11.5-NA) with paclitaxel and pazopanib (HR 0.60; 95% CI, 0.30-1.21; P=0.07). A higher rate of grade 3 and 4 toxicities were seen in the combination treatment arm, 54% vs. 22% (P=0.0052) (78). GOG-186J evaluated pazopanib in a slightly different population. In this phase II, placebo-controlled trial, 100 women with recurrent, measurable/evaluable EOC with 1-3 prior lines of therapy were randomized to weekly paclitaxel alone or in combination with pazopanib. Similarly, PFS was the primary endpoint, but was not extended relative to weekly paclitaxel (median PFS 7.5 vs. 6.2 months, HR 0.84; 90% CI, 0.57-1.22; P=0.2). RR was 32% vs. 23%, respectively. AEs, such as hypertension, were more common in the combination arm and more often led to treatment discontinuation (37% vs. 10%). However, more patients discontinued treatment on the control arm for disease progression (65% vs. 32%). While recording similar observations regarding toxicity with MITO-11, GOG-186J was discrepant regarding the efficacy endpoints. Differences in design (open-label vs. placebo), eligibility (1-2 vs. 1-3 prior treatment lines, prognostic factors), and sample size may be responsible for the different observations.
Sorafenib
A neoadjuvant phase II study for advanced stage EOC and large volume ascites evaluated sorafenib 400 mg twice daily with carboplatin/paclitaxel. The study was closed after four patients however due to life threatening toxicities (cardiac output failure, myocardial infarction, anastomotic leak) (79). In a placebo-controlled randomized phase II trial, sorafenib as single agent maintenance therapy in EOC showed no difference in the primary endpoint of PFS (median 12.7 vs. 15.7 months; hazard ratio 1.09; 95% CI, 0.72-1.63; P=0.655). More grade 3 toxicities were seen in the sorafenib group compared to placebo including hand-foot skin reactions and rash, and a high rate of dose reductions and early discontinuations were noted (80).
As a single agent in recurrent EOC (81), 24% of patients were progression-free at 6 months; yet, only 3% had a PR and multiple Grade 3 and 4 toxicities were documented. Sorafenib was also used in combination with topotecan (82) and with gemcitabine (83). Weekly gemcitabine with 400 mg twice daily of sorafenib was given in recurrent EOC. The primary endpoint of RR by RECIST criteria was not met; furthermore, dose reductions and grade 3 and 4 toxicities including primary lymphocytopenia and neutropenia were reported. In a phase II trial of sorafenib 400 mg daily and topotecan 3.5 mg/m2 weekly on days 1, 8, 15 of a 28-day cycle, a PR was achieved in 16.7% of patients. Grade 3 and 4 toxicities were reported including leukopenia/neutropenia (23%), thrombocytopenia (17%), and anemia (10%). A phase II study evaluated the combination of bevacizumab 5 mg/kg q2wk and sorafenib at a lower dose 200 mg bid days 1-5/week in patients with recurrent EOC (84). In 35 patients with no prior bevacizumab exposure, an 86% clinical RR was noted with 25.7% PRs and 60% with stable disease (SD) for greater than 4 months. About 54% of patients with prior bevacizumab treatment had SD. The therapy-related grade 3 and 4 AEs included hypertension (33%), DVT or PE (9%), and renal hemorrhage, perforation, anal fissure, and hand-foot syndrome (2% each). While sorafenib showed potential in these trials, the high rate of grade 3 toxicities raises questions about dosing and tolerance.
Trebananib
A phase III trial, TRINOVA-1, randomized patients to paclitaxel with placebo or with trebananib in patients with recurrent EOC who have had less than three platinum regimens or less than 12 months from the first platinum regimen. An improved RR was demonstrated in the combination group (29.8% vs. 38.4%, P=0.0071). PFS was 5.4 vs. 7.2 months (HR 0.66; 95% CI, 0.57-0.77; P=0.001) and OS was 17.3 vs. 19.0 months (HR 0.86; 95% CI, 0.69-1.08; P=0.19) (85). TRINOVA-2 and TRINOVA-3 are ongoing phase III studies. TRINOVA-2 (86) is comparing trebananib vs. placebo in combination with PLD in patients with recurrent EOC who received less than three cycles of chemotherapy or less than 12 months from the first platinum regimen. Frontline treatment is being studied in TRINOVA-3 (87). Patients are being randomized to standard therapy (carboplatin/paclitaxel) and placebo followed placebo maintenance or standard therapy with trebananib followed by trebananib maintenance for up to 18 months. Results are expected in 2016.
Vandetanib
Vandetanib was evaluated as a monotherapy in recurrent EOC. The study was terminated after 12 patients for lack of response or stabilizing disease beyond 6 months (88). Vandetanib was also studied in combination with chemotherapy. A phase I/II study of platinum resistant EOC evaluated vandetanib in combination with PLD. While a clinical response was seen (1 PR and 4 SD), 29% of patients stopped treatment secondary to toxicities including neutropenia, mucositis, and palmar-plantar erythrodysesthesia (89). Vandetanib was also investigated in a phase II trial comparing vandetanib and docetaxel to docetaxel alone. Unlike the prior trial with PLD, no significant toxicities were seen in combination with docetaxel; however no significant difference in median PFS was seen between the two arms [3.0 months (D + V) vs. 3.5 (D); HR: 0.99 (80% CI: 0.79-1.26)] (90).
VDAs: fosbretabulin
The possible synergistic combination of VDAs and anti-angiogenics has been studied in several phase I/II trials (91). In a nude mouse model, human clear cell renal tumors were treated with VDA alone and in combination with bevacizumab. A significantly greater tumor response was seen in the combination therapy groups (92). GOG-186I randomized 107 women with measurable/detectable recurrent EOC and 1-3 lines of prior therapy to bevacizumab or bevacizumab combined with the VDA, fosbretabulin. PFS, the primary endpoint, was significantly improved in the combination arm (median 7.3 vs. 4.8; HR 0.69; 90% CI, 0.47-1.00; P=0.049). Correspondingly, the RR was 36% and 28%, respectively. AEs (>grade 2) were more common in the combination arm, particularly hypertension (35% vs. 16%). The authors concluded that the combined anti-vascular approach was active, warranting further study as a novel, chemotherapy-free treatment strategy, but noted higher rates of AEs.
Future directions
As discussed earlier, when patients in ICON7 and GOG 218 were stratified based on clinical criteria, high risk patient populations were identified that would benefit more from treatment with bevacizumab. In AGO-OVAR 12, the opposite was found with the low risk group benefiting more from treatment with nintedanib. The ideal usage of these agents may not be based on debulking or stage but based on the tumor’s molecular ‘fingerprint’ (93,94). Serum samples from ICON7 were analyzed to determine biomarkers for response. Mesothelin, fms-like tyrosine kinase-4 and α1-acid glycoprotein were identified as markers of improved response. The biomarkers when combined with CA 125 were predictive of improved response in two cohorts (95). In one study, patient samples were divided into immunogenic and angiogenic signatures. The angiogenic subgroup treated with bevacizumab had improved PFS (17.4 vs. 12.3 months, P=0.003). However the immunogenic subtypes treated without bevacizumab had an improved PFS by 17.3 months (18.5 vs. 35.8 months, P=0.048) and OS (HR 2.37; 95% CI, 1.27-4.41, P=0.007). The second study used the subgroups identified by The Cancer Genome Atlas data (differentiated, immunoreactive, mesenchymal, and proliferative). The proliferative group showed the most effect with bevacizumab. These profiling techniques are promising but need validation before clinical adoption.
Conclusions
Novel agents that target angiogenesis include single and multiple pathway inhibitors. These agents have been evaluated as single agents or in combination with chemotherapy or other molecular therapies for frontline and recurrent EOC with mixed results. Bevacizumab, the most studied of these agents, has showed improved PFS in four randomized phase III studies. Based on the positive outcomes on PFS, bevacizumab is the first of the anti-angiogenic agents to be approved for the treatment of EOC by the FDA and the EU. Bevacizumab’s approval by the FDA for recurrent platinum resistant EOC will hopefully continue to encourage development and approval of novel molecular targets.
While bevacizumab has shown a positive effect on PFS, the ideal population and time for treatment as well as the dose and duration of treatment remain unclear. Some experts argue that the use of these agents should be earlier to provide the greatest potential for cure, while others cite the lack of demonstrated OS to date in this setting. The most impressive results in terms of hazard ratios have been demonstrated in the recurrent queues, yet others are concerned with increasing toxicities in non-curative settings. The optimal dose level for bevacizumab has also been debated as this variable impacts cost analysis. Most studies in ovarian cancer have used the 15 mg/kg dose, and this has been the standard in the United States until further data establishes that a lower dose has equivalent efficacy.
The role of combining anti-angiogenics in the maintenance setting appears promising but whether these agents should be administered concomitant with chemotherapy or only after is unclear based on the disparate study designs across agents. Another major issue is whether the inhibition of more than one angiogenic pathway will optimize the use of these agents, but this may come at the expense of increased toxicity. Further investigations to identify true synergies are indicated. Controlling toxicities and costs associated with these agents as well as identifying biomarkers to predict efficacy for these agents are high priority goals; nonetheless, these agents are a welcome addition to our clinical armamentarium for the treatment of women with ovarian cancer.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Franco Muggia and Eleonora Teplinsky) for the series “Epithelial Ovarian Cancer Treatment: Integrating Molecular Targeting” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: The series “Epithelial Ovarian Cancer Treatment: Integrating Molecular Targeting” was commissioned by the editorial office without any funding or sponsorship. Drs. Jackson and Davenport have no conflicts of interest to report. Dr. Herzog serves on consultant advisory board’s for Genentech, Morphotech, Vertex, and AstraZeneca. Dr. Coleman receives research funding from Janssen, EMDserono, Clovis, AstraZeneca, Millennium, Array, and Merck. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [PubMed]
- Colombo N, Peiretti M, Parma G, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21:v23-30. [PubMed]
- Katsumata N, Yasuda M, Isonishi S, et al. Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): a randomised, controlled, open-label trial. Lancet Oncol 2013;14:1020-6. [PubMed]
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med 1971;285:1182-6. [PubMed]
- Ide AG, Baker NH, Warren SL. Vascularization of the brown Pearce rabbit epithelioma transplant as seen in the transparent ear chamber. Am J Roentgenol 1939;42:891-9.
- Folkman J, Merler E, Abernathy C, et al. Isolation of a tumor factor responsible for angiogenesis. J Exp Med 1971;133:275-88. [PubMed]
- Ferrara N, Carver-Moore K, Chen H, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 1996;380:439-42. [PubMed]
- Bandiera E, Franceschini R, Specchia C, et al. Prognostic significance of vascular endothelial growth factor serum determination in women with ovarian cancer. ISRN Obstet Gynecol 2012;2012:245756.
- Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J Mol Med (Berl) 1999;77:527-43. [PubMed]
- Ferrara N. Role of vascular endothelial growth factor in the regulation of angiogenesis. Kidney Int 1999;56:794-814. [PubMed]
- Ferrara N. Vascular endothelial growth factor: molecular and biological aspects. Curr Top Microbiol Immunol 1999;237:1-30. [PubMed]
- Arjaans M, Oosting SF, Schroder CP, et al. Bevacizumab-induced vessel normalization hampers tumor uptake of antibodies--response. Cancer Res 2013;73:7147-8. [PubMed]
- Burger RA. Experience with bevacizumab in the management of epithelial ovarian cancer. J Clin Oncol 2007;25:2902-8. [PubMed]
- Randall LM, Monk BJ. Bevacizumab toxicities and their management in ovarian cancer. Gynecol Oncol 2010;117:497-504. [PubMed]
- Stewart MW. Aflibercept (VEGF Trap-eye): the newest anti-VEGF drug. Br J Ophthalmol 2012;96:1157-8. [PubMed]
- Stewart MW, Grippon S, Kirkpatrick P. Aflibercept. Nat Rev Drug Discov 2012;11:269-70. [PubMed]
- Lindahl P, Johansson BR, Leveen P, et al. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 1997;277:242-5. [PubMed]
- Oliner J, Min H, Leal J, et al. Suppression of angiogenesis and tumor growth by selective inhibition of angiopoietin-2. Cancer Cell 2004;6:507-16. [PubMed]
- Liu Z, Fan F, Wang A, et al. Dll4-Notch signaling in regulation of tumor angiogenesis. J Cancer Res Clin Oncol 2014;140:525-36. [PubMed]
- Williams CK, Li JL, Murga M, et al. Up-regulation of the Notch ligand Delta-like 4 inhibits VEGF-induced endothelial cell function. Blood 2006;107:931-9. [PubMed]
- Gridley T. Notch signaling during vascular development. Proc Natl Acad Sci U S A 2001;98:5377-8. [PubMed]
- Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol 2006;7:678-89. [PubMed]
- Sainson RC, Harris AL. Anti-Dll4 therapy: can we block tumour growth by increasing angiogenesis? Trends Mol Med 2007;13:389-95. [PubMed]
- Lu C, Bonome T, Li Y, et al. Gene alterations identified by expression profiling in tumor-associated endothelial cells from invasive ovarian carcinoma. Cancer Res 2007;67:1757-68. [PubMed]
- Hu W, Lu C, Dong HH, et al. Biological roles of the Delta family Notch ligand Dll4 in tumor and endothelial cells in ovarian cancer. Cancer Res 2011;71:6030-9. [PubMed]
- Uyttendaele H, Ho J, Rossant J, et al. Vascular patterning defects associated with expression of activated Notch4 in embryonic endothelium. Proc Natl Acad Sci U S A 2001;98:5643-8. [PubMed]
- Groeneweg JW, Foster R, Growdon WB, et al. Notch signaling in serous ovarian cancer. J Ovarian Res 2014;7:95. [PubMed]
- Mita MM, Sargsyan L, Mita AC, et al. Vascular-disrupting agents in oncology. Expert Opin Investig Drugs 2013;22:317-28. [PubMed]
- Porcù E, Bortolozzi R, Basso G, et al. Recent advances in vascular disrupting agents in cancer therapy. Future Med Chem 2014;6:1485-98. [PubMed]
- Randall LM, Burger RA, Nguyen H, et al. Outcome differences in patients with advanced epithelial ovarian, primary peritoneal and fallopian tube cancers treated with and without bevacizumab. Gynecol Oncol SGO Abstract Suppl: S34; SGO #80, 2013. (Abstract).
- Perren TJ, Swart AM, Pfisterer J, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 2011;365:2484-96. [PubMed]
- Oza A, Perren T, Swart A, et al. ICON7: Final overall survival results in GCIG phase III randomized trial of bevacizumab in women with newly diagnosed ovarian cancer. E J Cancer 2013;49:LBA:6.
- Rouzier R, Morice P, Floquet A, et al. A randomized, open-label, phase II study assessing the efficacy and the safety of bevacizumab in neoadjuvant therapy in patients with FIGO stage IIIc/IV ovarian, tubal, or peritoneal adenocarcinoma, initially unresectable. J Clin Oncol 2014;32:abstr TPS5614.
- González-Cao M, Viteri S, Díaz-Lagares A, et al. Preliminary results of the combination of bevacizumab and weekly Paclitaxel in advanced melanoma. Oncology 2008;74:12-6. [PubMed]
- Gonzalez-Martin A, Gladieff L, Tholander B, et al. Efficacy and safety results from OCTAVIA, a single-arm phase II study evaluating front-line bevacizumab, carboplatin and weekly paclitaxel for ovarian cancer. Eur J Cancer 2013;49:3831-8. [PubMed]
- Gonzalez-Martin A, Gladieff L, Tholander B, et al. Updated results from OCTAVIA (front-line bevacizumab, carboplatin and weekly paclitaxel therapy for ovarian cancer). Eur J Cancer 2014;50:862-3. [PubMed]
- Chan J, Brady M, Penson R, et al. Phase III trial of every-3-weeks paclitaxel versus dose dense weekly paclitaxel with carboplatin +/- bevacizumab in epithelial ovarian, peritoneal, fallopian tube cancer: GOG 262 (NCT0116712). Oral presentation at the 2013 European Society of Gynecological Oncology Annual Meeting, Liverpool (Accessed on October 23, 2013).
- Aghajanian C, Blank SV, Goff BA, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol 2012;30:2039-45. [PubMed]
- Pujade-Lauraine E, Hilpert F, Weber B, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol 2014;32:1302-8. [PubMed]
- Witteveen P, Lortholary A, Fehm T, et al. Final overall survival (OS) results from AURELIA, an open-label randomised phase III trial of chemotherapy (CT) with or without bevacizumab (BEV) for platinum-resistant recurrent ovarian cancer (OC). EJC 2013;49:LBA5.
- Chura JC, Van Iseghem K, Downs LS Jr, et al. Bevacizumab plus cyclophosphamide in heavily pretreated patients with recurrent ovarian cancer. Gynecol Oncol 2007;107:326-30. [PubMed]
- Garcia AA, Hirte H, Fleming G, et al. Phase II clinical trial of bevacizumab and low-dose metronomic oral cyclophosphamide in recurrent ovarian cancer: a trial of the California, Chicago, and Princess Margaret Hospital phase II consortia. J Clin Oncol 2008;26:76-82. [PubMed]
- Matulonis UA, Pereira L, Liu J, et al. Sequential bevacizumab and oral cyclophosphamide for recurrent ovarian cancer. Gynecol Oncol 2012;126:41-6. [PubMed]
- del Carmen MG, Micha J, Small L, et al. A phase II clinical trial of pegylated liposomal doxorubicin and carboplatin plus bevacizumab in patients with platinum-sensitive recurrent ovarian, fallopian tube, or primary peritoneal cancer. Gynecol Oncol 2012;126:369-74. [PubMed]
- Kudoh K, Takano M, Kouta H, et al. Effects of bevacizumab and pegylated liposomal doxorubicin for the patients with recurrent or refractory ovarian cancers. Gynecol Oncol 2011;122:233-7. [PubMed]
- Verschraegen CF, Czok S, Muller CY, et al. Phase II study of bevacizumab with liposomal doxorubicin for patients with platinum- and taxane-resistant ovarian cancer. Ann Oncol 2012;23:3104-10. [PubMed]
- Eisenhauer EL, Zanagnolo V, Cohn DE, et al. A phase II study of gemcitabine, carboplatin and bevacizumab for the treatment of platinum-sensitive recurrent ovarian cancer. Gynecol Oncol 2014;134:262-6. [PubMed]
- Wenham RM, Lapolla J, Lin HY, et al. A phase II trial of docetaxel and bevacizumab in recurrent ovarian cancer within 12 months of prior platinum-based chemotherapy. Gynecol Oncol 2013;130:19-24. [PubMed]
- Herzog TJ, Monk BJ, Rose PG, et al. A phase II trial of oxaliplatin, docetaxel, and bevacizumab as first-line therapy of advanced cancer of the ovary, peritoneum, and fallopian tube. Gynecol Oncol 2014;132:517-25. [PubMed]
- Ling HT, Muggia F, Speyer JL, et al. A phase II trial on the combination of bevacizumab and irinotecan in recurrent ovarian cancer. J Clin Oncol 2014;32:abstr 5564.
- McGonigle KF, Muntz HG, Vuky J, et al. Combined weekly topotecan and biweekly bevacizumab in women with platinum-resistant ovarian, peritoneal, or fallopian tube cancer: results of a phase 2 study. Cancer 2011;117:3731-40. [PubMed]
- Tillmanns TD, Lowe MP, Walker MS, et al. Phase II clinical trial of bevacizumab with albumin-bound paclitaxel in patients with recurrent, platinum-resistant primary epithelial ovarian or primary peritoneal carcinoma. Gynecol Oncol 2013;128:221-8. [PubMed]
- Ikeda Y, Takano M, Oda K, et al. Weekly administration of bevacizumab, gemcitabine, and oxaliplatin in patients with recurrent and refractory ovarian cancer: a preliminary result of 19 cases. Int J Gynecol Cancer 2013;23:355-60. [PubMed]
- Burger RA, Sill MW, Monk BJ, et al. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol 2007;25:5165-71. [PubMed]
- Cannistra SA, Matulonis UA, Penson RT, et al. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J Clin Oncol 2007;25:5180-6. [PubMed]
- Smerdel MP, Steffensen KD, Waldstrom M, et al. The predictive value of serum VEGF in multiresistant ovarian cancer patients treated with bevacizumab. Gynecol Oncol 2010;118:167-71. [PubMed]
- Lee JM, Sarosy GA, Annunziata CM, et al. Combination therapy: intermittent sorafenib with bevacizumab yields activity and decreased toxicity. Br J Cancer 2010;102:495-9. [PubMed]
- Nimeiri HS, Oza AM, Morgan RJ, et al. Efficacy and safety of bevacizumab plus erlotinib for patients with recurrent ovarian, primary peritoneal, and fallopian tube cancer: a trial of the Chicago, PMH, and California Phase II Consortia. Gynecol Oncol 2008;110:49-55. [PubMed]
- Chambers SK, Clouser MC, Baker AF, et al. Overexpression of tumor vascular endothelial growth factor A may portend an increased likelihood of progression in a phase II trial of bevacizumab and erlotinib in resistant ovarian cancer. Clin Cancer Res 2010;16:5320-8. [PubMed]
- Hagemann AR, Novetsky AP, Zighelboim I, et al. Phase II study of bevacizumab and pemetrexed for recurrent or persistent epithelial ovarian, fallopian tube or primary peritoneal cancer. Gynecol Oncol 2013;131:535-40. [PubMed]
- Tew WP, Sill M, McMeekin DS, et al. A randomized phase II trial of bevacizumab (BV) plus oral everolimus (EV) versus bevacizumab alone for recurrent or persistent epithelial ovarian (EOC), fallopian tube (FTC), or primary peritoneal cancer (PPC). J Clin Oncol 2014;32:abstr 5546.
- Ikeda Y, Takano M, Sasaki N, et al. Effect of weekly administration of bevacizumab, eribulin, and oxalilplatin in patients with heavily pretreated serous ovarian carcinoma. J Clin Oncol 2013;31:abstr e16506.
- Colombo N, Mangili G, Mammoliti S, et al. A phase II study of aflibercept in patients with advanced epithelial ovarian cancer and symptomatic malignant ascites. Gynecol Oncol 2012;125:42-7. [PubMed]
- Gotlieb WH, Amant F, Advani S, et al. Intravenous aflibercept for treatment of recurrent symptomatic malignant ascites in patients with advanced ovarian cancer: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Oncol 2012;13:154-62. [PubMed]
- Tew WP, Colombo N, Ray-Coquard I, et al. Intravenous aflibercept in patients with platinum-resistant, advanced ovarian cancer: results of a randomized, double-blind, phase 2, parallel-arm study. Cancer 2014;120:335-43. [PubMed]
- Coleman RL, Duska LR, Ramirez PT, et al. Phase 1-2 study of docetaxel plus aflibercept in patients with recurrent ovarian, primary peritoneal, or fallopian tube cancer. Lancet Oncol 2011;12:1109-17. [PubMed]
- Matulonis UA, Berlin S, Ivy P, et al. Cediranib, an oral inhibitor of vascular endothelial growth factor receptor kinases, is an active drug in recurrent epithelial ovarian, fallopian tube, and peritoneal cancer. J Clin Oncol 2009;27:5601-6. [PubMed]
- Hirte HW, Vidal L, Fleming GF, et al. A phase II study of cediranib (AZD2171) in recurrent or persistent ovarian, peritoneal or fallopian tube cancer: Final results of a PMH, Chicago and California consortia trial. J Clin Oncol 2008;26:5521.
- Raja FA, Griffin CL, Qian W, et al. Initial toxicity assessment of ICON6: a randomised trial of cediranib plus chemotherapy in platinum-sensitive relapsed ovarian cancer. Br J Cancer 2011;105:884-9. [PubMed]
- Raja F, Perren T, Embleton A, et al. Randomised double-blind phase III trial of cediranib (AZD 2171) in relapsed platinum sensitive ovarian cancer: results of the ICON6 trial. Int J Gynecol Cancer 2013;23:47.
- Liu J, Barry WT, Birrer MJ, et al. A randomized phase 2 trial comparing efficacy of the combination of the PARP inhibitor olaparib and the antiangiogenic cediranib against olaparib alone in recurrent platinum-sensitive ovarian cancer. J Clin Oncol 2014;32:abstr LBA5500.
- Ledermann JA, Hackshaw A, Kaye S, et al. Randomized phase II placebo-controlled trial of maintenance therapy using the oral triple angiokinase inhibitor BIBF 1120 after chemotherapy for relapsed ovarian cancer. J Clin Oncol 2011;29:3798-804. [PubMed]
- du Bois A, Kristensen G, Ray-Coquard I, et al. AGO-OVAR 12: a randomized placebo-controlled GCIG/ENGOT-intergroup phase III trial of standard frontline chemotherapy +/- nintedanib for advanced ovarian cancer. Int J Gynecol Cancer 2013;23:7.
- du Bois A, Vergote I, Wimberger P, et al. Open-label feasibility study of pazopanib, carboplatin, and paclitaxel in women with newly diagnosed, untreated, gynaecologic tumours: a phase I/II trial of the AGO study group. Br J Cancer 2012;106:629-32. [PubMed]
- Friedlander M, Hancock KC, Rischin D, et al. A Phase II, open-label study evaluating pazopanib in patients with recurrent ovarian cancer. Gynecol Oncol 2010;119:32-7. [PubMed]
- du Bois A, Floquet A, Kim JW, et al. Randomized, double-blind, phase III trial of pazopanib versus placebo in women who have not progressed after first-line chemotherapy for advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (AEOC): Results of an international Intergroup trial (AGO-OVAR16). J Clin Oncol 2013;31:abstr LBA5503.
- du Bois A, Floquet A, Kim JW, et al. Incorporation of pazopanib in maintenance therapy of ovarian cancer. J Clin Oncol 2014;32:3374-82. [PubMed]
- Pignata S, Larusso D, Scambia G, et al. MITO-11: A randomized multicenter phase II trial testing the addition of pazopanib to weekly paclitaxel in platinum-resistant or -refractory advanced ovarian cancer (AOC). J Clin Oncol 2014;32:abstr 5503^. Available online: http://meetinglibrary.asco.org/content/131237-144
- Pölcher M, Eckhardt M, Coch C, et al. Sorafenib in combination with carboplatin and paclitaxel as neoadjuvant chemotherapy in patients with advanced ovarian cancer. Cancer Chemother Pharmacol 2010;66:203-7. [PubMed]
- Herzog TJ, Scambia G, Kim BG, et al. A randomized phase II trial of maintenance therapy with Sorafenib in front-line ovarian carcinoma. Gynecol Oncol 2013;130:25-30. [PubMed]
- Matei D, Sill MW, Lankes HA, et al. Activity of sorafenib in recurrent ovarian cancer and primary peritoneal carcinomatosis: a gynecologic oncology group trial. J Clin Oncol 2011;29:69-75. [PubMed]
- Ramasubbaiah R, Perkins SM, Schilder J, et al. Sorafenib in combination with weekly topotecan in recurrent ovarian cancer, a phase I/II study of the Hoosier Oncology Group. Gynecol Oncol 2011;123:499-504. [PubMed]
- Welch SA, Hirte HW, Elit L, et al. Sorafenib in combination with gemcitabine in recurrent epithelial ovarian cancer: a study of the Princess Margaret Hospital Phase II Consortium. Int J Gynecol Cancer 2010;20:787-93. [PubMed]
- Lee JM, Annunziata CM, Hays JL, et al. A phase II study of intermittent sorafenib with bevacizumab (B) in B-naive and prior B-exposed epithelial ovarian cancer (EOC) patients. J Clin Oncol 2014;32:abstr 5553.
- Monk BJ, Poveda A, Vergote I, et al. Anti-angiopoietin therapy with trebananib for recurrent ovarian cancer (TRINOVA-1): a randomised, multicentre, double-blind, placebo-controlled phase 3 trial. Lancet Oncol 2014;15:799-808. [PubMed]
- TRINOVA-2: Trebananib in Ovarian Cancer-2. Available online: http://clinicaltrials.gov, accessed 14 Jan 2015.
- TRINOVA-3: A study of AMG 386 or AMG 386 Placebo in Combination With Paclitaxel and Carboplatin to Treat Ovarian Cancer. Available online: http://clinicaltrials.gov, accessed 14 Jan 2015.
- Annunziata CM, Walker AJ, Minasian L, et al. Vandetanib, designed to inhibit VEGFR2 and EGFR signaling, had no clinical activity as monotherapy for recurrent ovarian cancer and no detectable modulation of VEGFR2. Clin Cancer Res 2010;16:664-72. [PubMed]
- Harter P, Sehouli J, Kimmig R, et al. Addition of vandetanib to pegylated liposomal doxorubicin (PLD) in patients with recurrent ovarian cancer. A randomized phase I/II study of the AGO Study Group (AGO-OVAR 2.13). Invest New Drugs 2013;31:1499-504. [PubMed]
- Coleman RL, Moon J, Sood AK, et al. Randomised phase II study of docetaxel plus vandetanib versus docetaxel followed by vandetanib in patients with persistent or recurrent epithelial ovarian, fallopian tube or primary peritoneal carcinoma: SWOG S0904. Eur J Cancer 2014;50:1638-48. [PubMed]
- Cooney MM, van Heeckeren W, Bhakta S, et al. Drug insight: vascular disrupting agents and angiogenesis--novel approaches for drug delivery. Nat Clin Pract Oncol 2006;3:682-92. [PubMed]
- Siemann DW, Shi W. Dual targeting of tumor vasculature: combining Avastin and vascular disrupting agents (CA4P or OXi4503). Anticancer Res 2008;28:2027-31. [PubMed]
- Winterhoff BJ, Kommoss S, Oberg AL, et al. Bevacizumab and improvement of progression-free survival (PFS) for patients with the mesenchymal molecular subtype of ovarian cancer. J Clin Oncol 2014;32:abstr 5509.
- Gourley C, McCavigan A, Perren T, et al. Molecular subgroup of high-grade serous ovarian cancer (HGSOC) as a predictor of outcome following bevacizumab. J Clin Oncol 2014;32:abstr 5502.
- Collinson F, Hutchinson M, Craven RA, et al. Predicting response to bevacizumab in ovarian cancer: a panel of potential biomarkers informing treatment selection. Clin Cancer Res 2013;19:5227-39. [PubMed]


