
Expression and functions of semaphorins in cancer
The semaphorin family
The semaphorin family includes a large number of secreted, transmembrane, and GPI-linked proteins, and has been reported to play pivotal roles in directing the development and morphogenesis of the nervous system (1-3). The common feature of all semaphorins is an N-terminal region of ~500 highly disulfide-linked amino acids, named the “sema” domain, but they can be further categorized into eight subclasses based on the distinctive structural domains in their C-termini (4). Classes 1 and 2 exist only in invertebrate organisms, and classes 3, 4, 6, and 7 are distributed over vertebrate genomes (5). Notably, class 5 is observed both in vertebrates and invertebrates. The class 5 semaphorins found in viruses are very similar to class 7 members (6). The widespread distribution of semaphorins across all species suggests the importance of their involvement in biological function.
Biological functions of semaphorins
Many primary functional studies have indicated that semaphorins are dominant axon repulsive or attractive molecules (7-10). Through interactions with and signaling to their downstream receptors, such as plexins and neuropilins (Nrps), semaphorins are able to control the assembly of the cytoskeleton and determine the direction of neuronal cell growth. The factors influencing whether semaphorins act as chemoattractants or chemorepellents are myriad, including the spatial distribution of semaphorins, their surrounding microenvironments, and the cell types present. A recent study also indicated that the bi-functional axon guidance feature of semaphorins may result from activation of distinct domains (11). For example, the sema domain of SEMA5A mediates the repulsion of developing motor axon growth and branching, but the thrombospondin repeat domain enhances the proliferation of axons into the ventral myotome (11). Such complicated bi-functional activities have been reported in several semaphorins, but the details of the switching mechanisms remain to be elucidated (12).
Because semaphorins regulate the growth and proliferation of neurons, the relationships between semaphorins and cytoskeleton units have been intensely studied. In this regard, SEMA7A promotes the outgrowth of central and peripheral axons by interacting with the integrin receptor and mitogen-activated protein kinase (MAPK) pathways (13). Also, class 3 semaphorins are major players that respond to mediate neuronal growth cone collapses through de-polymerization of F-actin assembly (14). In addition to their dominant role in neuronal guidance, semaphorins also participate in many other important cellular functions, such as the immune response and angiogenesis (15-17). Three subclasses of semaphorins (classes 3, 4, and 7) possess an immunoglobulin-like domain, and thus a growing body of evidence has reported their interactions with immune and plexin receptors (18,19). For instance, the protein product of SEMA4D, CD100, was the first semaphorin to be reported in activating and regulating the differentiation of T- and B-cells through a tyrosine phosphatase, CD45 (15). The expression of SEMA7A on activated T-cells may induce macrophages to generate cytokines responding to inflammation (20,21). Anti-angiogenic activities have been reported for SEMA3A and SEMA3E by counteracting vascular endothelial growth factor (VEGF) function or leading another signaling cascade mechanism (22). SEMA3A binding to its downstream member, Nrp1, may interfere with vascular patterning by suppressing integrin function (23), and SEMA3E may inhibit the functional effects of VEGF through enhancing the expression of its receptor, plexin-D1, and a small GTPase, RhoJ (24). Although explorations of the detailed mechanisms about how semaphorins are involved in angiogenic processes are ongoing, the emerging importance of them has turned these genes into potential drug targets in cancer therapy.
Semaphorins are important regulators in cancer cells from the perspective of both their biological functions and their structural features. Class 3 semaphorins have been widely reported as affecting several solid cancers (25-27). For instance, the expression level of SEMA3A has been shown to be a negative indicator of the invasiveness and metastatic ability of lung cancer cells (28). SEMA3B may antagonize the autocrine activity driven by VEGF and thus trigger apoptosis in lung and breast cancers (29). The sema domain in semaphorins shows high sequence homology with plexins and the extracellular domain of plasminogen-related growth factor (PRGF) receptors, which include two famous oncogenes, MET and RON (18). After binding to PRGFs, the intracellular tyrosine kinase and C-terminal domains of the receptors may become phosphorylated to initiate the signal transduction pathways in regulating their downstream enzyme activity. For example, such phosphorylation of MET has been demonstrated to be an essential step in activating the hepatocyte growth factor (HGF) signal when mouse embryos develop (30). In epithelial cells, the binding of SEMA4D to plexin-B1 promotes the phosphorylated form of Met, and all of them are associated in a complex to control the complicated processes of invasive growth (31).
Expression of semaphorins and their associated receptors in cancer
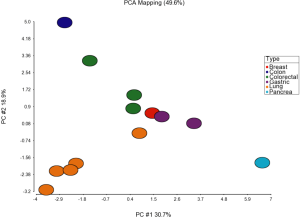
To evaluate the expression levels of semaphorins in multiple cancer types, we retrieved 13 mRNA microarray datasets with paired tumor and normal tissues from the same individual in the Gene Expression Omnibus (GEO) (32). The characteristics of each dataset are summarized in Table 1 (33-44). Approximately 450 pairs of samples were investigated within 6 different cancer types, and the relative ratios between cancer and normal tissues of 32 selected genes, including semaphorins, plexins and neuropilins, were assessed (Table 2). The expression levels of several genes were significantly (P-values <10-5) different in tumor tissues, and the expression profiles of these genes were depicted using hierarchical clustering with the average linkage method (Figure 1). As expected, a well-known tumor suppressor gene, SEMA3B, was universally down-regulated in half of the microarray datasets, consistent with the notion that semaphorins may serve as important regulators in driving transcriptional changes in tumor cells. Other semaphorins, in contrast, had different expression patterns in different cancer types (Figure 1). For example, SEMA5A was significantly down-regulated (by ~60%) in all five lung adenocarcinoma datasets. However, such down-regulation was not observed in other cancer types, and up-regulation was observed in gastric cancer. To further evaluate whether the expression of semaphorins and their receptors was correlated with specific cancer types, principal component analysis (PCA) was performed using the expression ratios of these 32 genes (Figure 2). Most of the lung adenocarcinoma samples aggregated in the lower left corner, whereas cases with colorectal cancer grouped around the center. This suggestes that expression patterns of semaphorin genes in cancer cells may be tissue specific. Therefore, to understand the regulatory mechanism of semaphorins in different transformed cells, a large-scale meta-analysis is needed to evaluate the association of semaphorins with different cancers.

Full table
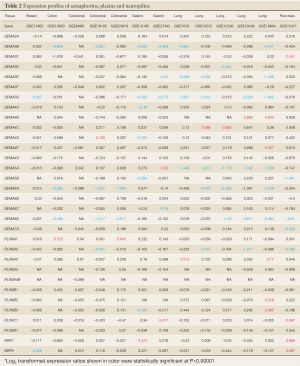
Full table

Biological functions of semaphorin classes 4-7 in cancers
A growing body of studies has evaluated the physiological roles of semaphorins and their associated receptors in cancer cells (45-47). The possible tumorigenic effects driven by the members of semaphorin class 3 have been comprehensively reviewed in several recent reports (1,22,48-50). Therefore, in the following paragraphs, we focus on the other four semaphorin classes (4-7). Their features in different cell types are summarized in Table 3.
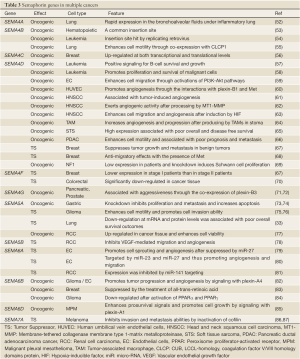
Full table
Semaphorin class 4
SEMA4A is generally regarded as an important regulator in the immune system (87,88). Since the increase of SEMA4A could be rapidly detected in the bronchoalveolar fluids of inflammatory lung, it has been characterized as a potential biomarker for identifying airway diseases with inflammation (51). As reported in several reviews, chronic inflammation is a causative factor leading to lung cancer (89-91), and thus whether the protein expression of SEMA4A can be used as a biomarker of early-stage lung carcinoma needs further investigation.
SEMA4B was reported as a potential proto-oncogene because SEMA4B is a common insertion site in hematopoietic cancer in humans and in a myeloproliferative disorder-like myeloid mouse strain (52,53). In addition, SEMA4B enhances cell motility through its coexpression with CLCP1 (CUB, LCCL-homology, coagulation factor V/VIII homology domains protein) in lung cancer cells (54) and thus more studies are required to investigate its tumorigenic activity.
The expression of SEMA4C was up-regulated at both the transcriptional and the translational levels in lymphatic endothelial cells derived from breast cancer tissues (55), indicating that SEMA4C may participate in the tumorigenesis of breast cancer.
A majority of the literature on SEMA4D indicates it has oncogenic effects in several tumor types, but a few studies also reported SEMA4D as tumor suppressor. A full elucidation of its function requires further investigation. Here, we summarize the signaling pathway of SEMA4D for carcinogenesis, based on published reports (Figure 3). First, a study showed that the interactions between CD38 and CD31 were upstream modulators of increased expression of SEMA4D (alias CD100), which enhanced B-cell growth and survival (56). Next, the downstream signaling cascade induced by SEMA4D and its high-affinity receptor, plexin-B1, elicits proliferation and survival signals in both normal and chronic lymphocytic leukemia (CLL) B-lymphocytes, while its major regulation mechanism was attributed to where the receptor was expressed (57). Three major signaling mechanisms downstream to plexin-B1 expression have been reported as key players in promoting angiogenesis and invasive growth: the c-Met pathway, Rho-initiated pathways, and the phosphatidylinositol 3-kinase-Akt (PI3K-AKT) pathway (16,31,58-60). Therefore, SEMA4D and plexin-B1 have been extensively studied to evaluate whether they could be used as potential drug targets for leukemia and lymphoma cells.
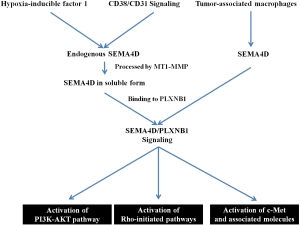
In addition to plexin-B1 signaling in leukemia and lymphoma cells, two possible mechanisms have been reported for the activity of SEMA4D in regulating tumor-induced angiogenesis in head and neck squamous cell carcinoma (HNSCC) cell lines (61,62). The first involved processing of SEMA4D to its soluble form by the membrane-tethered collagenase membrane type 1-matrix metalloproteinase (MT1-MMP) (61). The second mechanism was an increase in the expression of SEMA4D through activation of its upstream hypoxia response elements, most notably hypoxia-inducible factor 1 (62). Lastly, in addition to the endogenous SEMA4D, the tumor-associated macrophages within the surrounding stroma cells have been demonstrated as a major source of SEMA4D and thus may enhance tumor angiogenesis and progression (63).
High expression of SEMA4D was associated with poor survival in soft tissue sarcoma (64) and pancreatic ductal adenocarcinoma (65). On the contrary, another study revealed the expression of SEMA4D was significantly suppressed in grade III breast carcinoma, suggesting a protective effect of SEMA4D in benign tumors (66). Yet another report indicated that SEMA4D possesses both pro-migratory and anti-migratory abilities in breast cancer depending on the presence of ERBB2 and MET (67). Therefore, the regulation and signaling of SEMA4D deserve further investigation to elucidate its functional roles in cancer.
Similar to SEMA4D, contradictory effects of SEMA4F were reported in different cancers (66,68). Down-regulation of SEMA4F was widely observed in human neurofibroma, and knockdown of SEMA4F promoted proliferation of Schwann cells, suggesting its potential role as a tumor suppressor (68). Alternatively, SEMA4F may display oncogenic effects in breast cancer due to its higher expression in grade II tumor samples as compared to grade I (66). Additional studies are required to address whether this discrepancy could be attributed to the different tissue types.
Regarding SEMA4G, the expression of SEMA4G was significantly down-regulated in colorectal cancer tissues, which potentiates its role as a tumor suppressor (69).
Semaphorin class 5
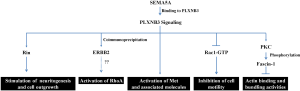
Similar to the seemingly contradictory effects mediated by SEMA4D, SEMA5A has been reported as a bi-functional molecule, acting as both oncogene and tumor suppressor in different types of cancer. The biological effects of SEMA5A from previous studies are summarized in Figure 4. High expression levels of SEMA5A and its receptor, plexin-B3, were associated with aggressiveness in pancreatic and prostate cancers (70). Their expressions were then found to be a driving source contributing to invasion, migration, proliferation, and tumor growth in gastric and pancreatic cancers (71-73,92). Two in vitro studies further indicated that expression of SEMA5A resulted in reduced apoptosis rates in endothelial and pancreatic cancer cells (72,93), suggesting a role in promoting tumor growth. Moreover, plexin-B3 was able to enhance neuritogenesis and cell outgrowth by interacting with Rin (Ras-like protein expressed in neurons) (94). Functional and coimmunoprecipitation assays revealed the association of plexin-B3 with MET and ERBB2 (95,96), and thus SEMA5A may induce invasive growth through the signaling pathways of them. On the other hand, SEMA5A has been found to be significantly suppressed in several cancer tissues, including colon (97), lung (33), and high-grade astrocytoma (74). Conversely, SEMA5A not only showed inhibitory effects on cell motility and invasiveness through inactivation of Rac1 GTPase, but also suppressed actin binding and bundling activities by elevation of protein kinase C (PKC) expression and fascin-1 phosphorylation level in glioma cells (74,75). Furthermore, down-regulation of SEMA5A was observed in lung cancer specimens, and was associated with poor overall survival outcomes at both transcriptional and translational levels (33). However, the genomic cytoband of SEMA5A, 5p15, often showed copy number amplifications in lung cancer samples (98-100), indicating that further investigation is warranted to elucidate its biological effects in lung cancer. An upstream conditional switch may exist to regulate the downstream signaling cascades.
SEMA5B was significantly up-regulated in clear cell renal cell carcinoma (ccRCC), and its knockdown was able to suppress cell viability (76). Very few studies have focused on the function of SEMA5C, and only one report has suggested it may be involved in regulating cell migration in the Drosophila embryo (101).
Semaphorin class 6
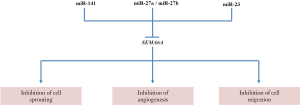
SEMA6A is regarded as an important tumor suppressor due to its critical functions in regulating cell sprouting, angiogenesis, and migration. The interactions of SEMA6A are summarized in Figure 5. SEMA6A has been identified as a dominant inhibitor of angiogenesis and VEGF-related tumor formation in several renal tumor tissues through the signaling of its extracellular domain (77). Recent reports indicated that tumor tissues might use different miRNAs to suppress the expression of SEMA6A (78-80). For instance, miR-141 was shown to inhibit SEMA6A using a transfection assay in renal cell carcinoma (80), and both miR-27a and miR-27b were able to lower the expression of SEMA6A through direct targeting in endothelial cells (78). The inhibitory effects of SEMA6A on migration and angiogenic activities of endothelial cells were impaired by miR-27 and miR-23 expressions, whose downstream signaling are mediated by controlling the phosphorylation levels of VEGFR2 and ERK1/2 in response to VEGF (79).
Opposite to the tumor suppressive effects of SEMA6A, a recent study showed that the signaling of SEMA6B and its receptor, plexin-A4, is able to enhance tumor progression and angiogenesis by increasing the expression of VEGF and bFGF in endothelial cells and a glioblastoma cell line, U87MG (81). An in vitro study also revealed the oncogenic effect of SEMA6B, whose expression was down-regulated in two glioma cell lines exposed to an anti-tumor drug, all-trans-retinoic acid (82). Moreover, activation of two well-known anti-angiogenic factors, peroxisome proliferator-activated receptors alpha and gamma (PPARα, PPARγ) (102), strongly reduced the expression of SEMA6B, but their family member with angiogenic effects, PPARβ, showed no effect on SEMA6B (83). Therefore, these results suggested SEMA6B might be an important node in transducing signals to activate angiogenic activity.
Most of the research about SEMA6D has focused on its regulatory role in cytoskeleton organization and the immune response (103,104). One report indicated that SEMA6D, along with its receptor, plexin-A1, enhanced pro-survival signals and anchorage-independent growth of malignant pleural mesothelioma cells (84). Unexpectedly, the survival-related signaling driven by SEMA6D was found to be associated with the transcriptional NFKB activity via the phosphorylation of VEGFR2 (84), elucidating a new pathway related to semaphorins. Furthermore, SEMA6D was significantly up-regulated at both the transcriptional and translational levels in gastric carcinoma (105), consistent with its oncogenic effects during tumorigenesis.
Semaphorin class 7
The signaling of SEMA7A and plexin-C1 has been demonstrated as an effective inhibitor of cofilin, whose activation was deemed as an important feature of tumor progression in melanoma (85,106). However, the expression of plexin-C1 is usually diminished during melanoma metastasis (85), which may lead to enhancement of melanoma invasion and metastasis abilities (86).
Conclusions
Although semaphorin genes were initially identified as key members in regulating the growth and migration of neuron cells, a growing body of evidence has strongly demonstrated their association with different types of cancer. It may be that the mechanisms used to control cytoskeleton assembly are also utilized in cancer cells. This hypothesis is supported by the fact that most of the functions related to semaphorins are associated with cell invasion, migration and motility. A review of the many available study reports provides a better understanding of their biological effects; however, challenges arise when integrating the results of the same gene obtained from different laboratories and distinct cancer types. Several semaphorins, such as SEMA5A, displayed both oncogenic and tumor suppressive effects in multiple cancers, a finding that highlights the two major issues needing to be addressed in the future. The first is the question of whether the same semaphorin performs contradictory functions in different cancer tissues, that is, whether its effects are tissue-specific. The second is the need to identify the switch, if it exists, that controls the transition from a tumor suppressor to an oncogene. The clearer our understanding of the role semaphorins play in tumor cells, the greater the possibility that we will be able to identify effective drug targets in cancer therapy.
Acknowledgments
We thank Melissa Stauffer, Ph.D., for editing the manuscript.
Funding: This research was supported in part by DOH98-TD-B-111-001 from the Department of Health, NSC98-2314-B-002-065-MY3 and NSC100-2314-B-002-054- from National Science Council, Taiwan, and 100R0001 from the Center of Genomic Medicine, National Taiwan University, Taiwan, ROC.】
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2012.04.04). EYC serves as the Editor-in-Chief of Translational Cancer Research. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Potiron VA, Roche J, Drabkin HA. Semaphorins and their receptors in lung cancer. Cancer Lett 2009;273:1-14. [PubMed]
- Chédotal A, Kerjan G, Moreau-Fauvarque C. The brain within the tumor: new roles for axon guidance molecules in cancers. Cell Death Differ 2005;12:1044-56. [PubMed]
- Luo L. Actin cytoskeleton regulation in neuronal morphogenesis and structural plasticity. Annu Rev Cell Dev Biol 2002;18:601-35. [PubMed]
- . Unified nomenclature for the semaphorins/collapsins. Semaphorin Nomenclature Committee. Cell 1999;97:551-2. [PubMed]
- Capparuccia L, Tamagnone L. Semaphorin signaling in cancer cells and in cells of the tumor microenvironment--two sides of a coin. J Cell Sci 2009;122:1723-36. [PubMed]
- Yazdani U, Terman JR. The semaphorins. Genome Biol 2006;7:211. [PubMed]
- Rohm B, Ottemeyer A, Lohrum M, et al. Plexin/neuropilin complexes mediate repulsion by the axonal guidance signal semaphorin 3A. Mech Dev 2000;93:95-104. [PubMed]
- Pasterkamp RJ, Verhaagen J. Semaphorins in axon regeneration: developmental guidance molecules gone wrong? Philos Trans R Soc Lond B Biol Sci 2006;361:1499-511. [PubMed]
- Nakamura F, Kalb RG, Strittmatter SM. Molecular basis of semaphorin-mediated axon guidance. J Neurobiol 2000;44:219-29. [PubMed]
- Bagnard D, Lohrum M, Uziel D, et al. Semaphorins act as attractive and repulsive guidance signals during the development of cortical projections. Development 1998;125:5043-53. [PubMed]
- Hilario JD, Rodino-Klapac LR, Wang C, et al. Semaphorin 5A is a bifunctional axon guidance cue for axial motoneurons in vivo. Dev Biol 2009;326:190-200. [PubMed]
- Derijck AA, Van Erp S, Pasterkamp RJ. Semaphorin signaling: molecular switches at the midline. Trends Cell Biol 2010;20:568-76. [PubMed]
- Pasterkamp RJ, Peschon JJ, Spriggs MK, et al. Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature 2003;424:398-405. [PubMed]
- Bielenberg DR, Shimizu A, Klagsbrun M. Semaphorin-induced cytoskeletal collapse and repulsion of endothelial cells. Methods Enzymol 2008;443:299-314. [PubMed]
- Potiron V, Nasarre P, Roche J, et al. Semaphorin signaling in the immune system. Adv Exp Med Biol 2007;600:132-44. [PubMed]
- Basile JR, Barac A, Zhu T, et al. Class IV semaphorins promote angiogenesis by stimulating Rho-initiated pathways through plexin-B. Cancer Res 2004;64:5212-24. [PubMed]
- Casazza A, Fazzari P, Tamagnone L. Semaphorin signals in cell adhesion and cell migration: functional role and molecular mechanisms. Adv Exp Med Biol 2007;600:90-108. [PubMed]
- Comoglio PM, Tamagnone L, Boccaccio C. Plasminogen-related growth factor and semaphorin receptors: a gene superfamily controlling invasive growth. Exp Cell Res 1999;253:88-99. [PubMed]
- O'Connor BP, Ting JP. The evolving role of semaphorins and plexins in the immune system: Plexin-A1 regulation of dendritic cell function. Immunol Res 2008;41:217-22. [PubMed]
- Suzuki K, Kumanogoh A, Kikutani H. Semaphorins and their receptors in immune cell interactions. Nat Immunol 2008;9:17-23. [PubMed]
- Tamagnone L, Artigiani S, Chen H, et al. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell 1999;99:71-80. [PubMed]
- Sakurai A, Doci C, Gutkind JS. Semaphorin signaling in angiogenesis, lymphangiogenesis and cancer. Cell Res 2012;22:23-32. [PubMed]
- Maione F, Molla F, Meda C, et al. Semaphorin 3A is an endogenous angiogenesis inhibitor that blocks tumor growth and normalizes tumor vasculature in transgenic mouse models. J Clin Invest 2009;119:3356-72. [PubMed]
- Fukushima Y, Okada M, Kataoka H, et al. Sema3E-PlexinD1 signaling selectively suppresses disoriented angiogenesis in ischemic retinopathy in mice. J Clin Invest 2011;121:1974-85. [PubMed]
- Clarhaut J, Roche J, Drabkin HA. Semaphorins in lung cancer. J Thorac Oncol 2006;1:203-4. [PubMed]
- Bagci T, Wu JK, Pfannl R, et al. Autocrine semaphorin 3A signaling promotes glioblastoma dispersal. Oncogene 2009;28:3537-50. [PubMed]
- Neufeld G, Kessler O. The semaphorins: versatile regulators of tumour progression and tumour angiogenesis. Nat Rev Cancer 2008;8:632-45. [PubMed]
- Shih JY, Yang SC, Hong TM, et al. Collapsin response mediator protein-1 and the invasion and metastasis of cancer cells. J Natl Cancer Inst 2001;93:1392-400. [PubMed]
- Castro-Rivera E, Ran S, Thorpe P, et al. Semaphorin 3B (SEMA3B) induces apoptosis in lung and breast cancer, whereas VEGF165 antagonizes this effect. Proc Natl Acad Sci U S A 2004;101:11432-7. [PubMed]
- Bardelli A, Longati P, Gramaglia D, et al. Gab1 coupling to the HGF/Met receptor multifunctional docking site requires binding of Grb2 and correlates with the transforming potential. Oncogene 1997;15:3103-11. [PubMed]
- Giordano S, Corso S, Conrotto P, et al. The semaphorin 4D receptor controls invasive growth by coupling with Met. Nat Cell Biol 2002;4:720-4. [PubMed]
- Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 2002;30:207-10. [PubMed]
- Lu TP, Tsai MH, Lee JM, et al. Identification of a novel biomarker, SEMA5A, for non-small cell lung carcinoma in nonsmoking women. Cancer Epidemiol Biomarkers Prev 2010;19:2590-7. [PubMed]
- Su LJ, Chang CW, Wu YC, et al. Selection of DDX5 as a novel internal control for Q-RT-PCR from microarray data using a block bootstrap re-sampling scheme. BMC Genomics 2007;8:140. [PubMed]
- Landi MT, Dracheva T, Rotunno M, et al. Gene expression signature of cigarette smoking and its role in lung adenocarcinoma development and survival. PLoS One 2008;3:e1651.
- Xi L, Feber A, Gupta V, et al. Whole genome exon arrays identify differential expression of alternatively spliced, cancer-related genes in lung cancer. Nucleic Acids Res 2008;36:6535-47. [PubMed]
- Hou J, Aerts J, den Hamer B, et al. Gene expression-based classification of non-small cell lung carcinomas and survival prediction. PLoS One 2010;5:e10312 [PubMed]
- Lasanthi-Kudahettige R, Magneschi L, Loreti E, et al. Transcript profiling of the anoxic rice coleoptile. Plant Physiol 2007;144:218-31. [PubMed]
- Jiang X, Tan J, Li J, et al. DACT3 is an epigenetic regulator of Wnt/beta-catenin signaling in colorectal cancer and is a therapeutic target of histone modifications. Cancer Cell 2008;13:529-41. [PubMed]
- Badea L, Herlea V, Dima SO, et al. Combined gene expression analysis of whole-tissue and microdissected pancreatic ductal adenocarcinoma identifies genes specifically overexpressed in tumor epithelia. Hepatogastroenterology 2008;55:2016-27. [PubMed]
- Pau Ni IB, Zakaria Z, Muhammad R, et al. Gene expression patterns distinguish breast carcinomas from normal breast tissues: the Malaysian context. Pathol Res Pract 2010;206:223-8. [PubMed]
- Matsuyama T, Ishikawa T, Mogushi K, et al. MUC12 mRNA expression is an independent marker of prognosis in stage II and stage III colorectal cancer. Int J Cancer 2010;127:2292-9. [PubMed]
- Cui J, Chen Y, Chou WC, et al. An integrated transcriptomic and computational analysis for biomarker identification in gastric cancer. Nucleic Acids Res 2011;39:1197-207. [PubMed]
- Cui J, Li F, Wang G, et al. Gene-expression signatures can distinguish gastric cancer grades and stages. PLoS One 2011;6:e17819 [PubMed]
- Müller MW, Giese NA, Swiercz JM, et al. Association of axon guidance factor semaphorin 3A with poor outcome in pancreatic cancer. Int J Cancer 2007;121:2421-33. [PubMed]
- Rolny C, Capparuccia L, Casazza A, et al. The tumor suppressor semaphorin 3B triggers a prometastatic program mediated by interleukin 8 and the tumor microenvironment. J Exp Med 2008;205:1155-71. [PubMed]
- Kusy S, Nasarre P, Chan D, et al. Selective suppression of in vivo tumorigenicity by semaphorin SEMA3F in lung cancer cells. Neoplasia 2005;7:457-65. [PubMed]
- Neufeld G, Sabag AD, Rabinovicz N, et al. Semaphorins in angiogenesis and tumor progression. Cold Spring Harb Perspect Med 2012;2:a006718 [PubMed]
- Staton CA. Class 3 semaphorins and their receptors in physiological and pathological angiogenesis. Biochem Soc Trans 2011;39:1565-70. [PubMed]
- Blanc V, Nariculam J, Munson P, et al. A role for class 3 semaphorins in prostate cancer. Prostate 2011;71:649-58. [PubMed]
- Smith EP, Shanks K, Lipsky MM, et al. Expression of neuroimmune semaphorins 4A and 4D and their receptors in the lung is enhanced by allergen and vascular endothelial growth factor. BMC Immunol 2011;12:30. [PubMed]
- Li Z, Kustikova OS, Kamino K, et al. Insertional mutagenesis by replication-deficient retroviral vectors encoding the large T oncogene. Ann N Y Acad Sci 2007;1106:95-113. [PubMed]
- Akagi K, Suzuki T, Stephens RM, et al. RTCGD: retroviral tagged cancer gene database. Nucleic Acids Res 2004;32:D523-7. [PubMed]
- Nagai H, Sugito N, Matsubara H, et al. CLCP1 interacts with semaphorin 4B and regulates motility of lung cancer cells. Oncogene 2007;26:4025-31. [PubMed]
- Wu M, Han L, Shi Y, et al. Development and characterization of a novel method for the analysis of gene expression patterns in lymphatic endothelial cells derived from primary breast tissues. J Cancer Res Clin Oncol 2010;136:863-72. [PubMed]
- Deaglio S, Vaisitti T, Bergui L, et al. CD38 and CD100 lead a network of surface receptors relaying positive signals for B-CLL growth and survival. Blood 2005;105:3042-50. [PubMed]
- Granziero L, Circosta P, Scielzo C, et al. CD100/Plexin-B1 interactions sustain proliferation and survival of normal and leukemic CD5+ B lymphocytes. Blood 2003;101:1962-9. [PubMed]
- Basile JR, Afkhami T, Gutkind JS. Semaphorin 4D/plexin-B1 induces endothelial cell migration through the activation of PYK2, Src, and the phosphatidylinositol 3-kinase-Akt pathway. Mol Cell Biol 2005;25:6889-98. [PubMed]
- Conrotto P, Valdembri D, Corso S, et al. Sema4D induces angiogenesis through Met recruitment by Plexin B1. Blood 2005;105:4321-9. [PubMed]
- Basile JR, Castilho RM, Williams VP, et al. Semaphorin 4D provides a link between axon guidance processes and tumor-induced angiogenesis. Proc Natl Acad Sci U S A 2006;103:9017-22. [PubMed]
- Basile JR, Holmbeck K, Bugge TH, et al. MT1-MMP controls tumor-induced angiogenesis through the release of semaphorin 4D. J Biol Chem 2007;282:6899-905. [PubMed]
- Sun Q, Zhou H, Binmadi NO, et al. Hypoxia-inducible factor-1-mediated regulation of semaphorin 4D affects tumor growth and vascularity. J Biol Chem 2009;284:32066-74. [PubMed]
- Sierra JR, Corso S, Caione L, et al. Tumor angiogenesis and progression are enhanced by Sema4D produced by tumor-associated macrophages. J Exp Med 2008;205:1673-85. [PubMed]
- Ch'ng E, Tomita Y, Zhang B, et al. Prognostic significance of CD100 expression in soft tissue sarcoma. Cancer 2007;110:164-72. [PubMed]
- Kato S, Kubota K, Shimamura T, et al. Semaphorin 4D, a lymphocyte semaphorin, enhances tumor cell motility through binding its receptor, plexinB1, in pancreatic cancer. Cancer Sci 2011;102:2029-37. [PubMed]
- Gabrovska PN, Smith RA, Tiang T, et al. Semaphorin-plexin signalling genes associated with human breast tumourigenesis. Gene 2011;489:63-9. [PubMed]
- Swiercz JM, Worzfeld T, Offermanns S. ErbB-2 and met reciprocally regulate cellular signaling via plexin-B1. J Biol Chem 2008;283:1893-901. [PubMed]
- Parrinello S, Noon LA, Harrisingh MC, et al. NF1 loss disrupts Schwann cell-axonal interactions: a novel role for semaphorin 4F. Genes Dev 2008;22:3335-48. [PubMed]
- Wang X, Zbou C, Qiu G, et al. Screening of new tumor suppressor genes in sporadic colorectal cancer patients. Hepatogastroenterology 2008;55:2039-44. [PubMed]
- Sadanandam A, Varney ML, Kinarsky L, et al. Identification of functional cell adhesion molecules with a potential role in metastasis by a combination of in vivo phage display and in silico analysis. OMICS 2007;11:41-57. [PubMed]
- Sadanandam A, Varney ML, Singh S, et al. High gene expression of semaphorin 5A in pancreatic cancer is associated with tumor growth, invasion and metastasis. Int J Cancer 2010;127:1373-83. [PubMed]
- Pan G, Lv H, Ren H, et al. Elevated expression of semaphorin 5A in human gastric cancer and its implication in carcinogenesis. Life Sci 2010;86:139-44. [PubMed]
- Pan GQ, Ren HZ, Zhang SF, et al. Expression of semaphorin 5A and its receptor plexin B3 contributes to invasion and metastasis of gastric carcinoma. World J Gastroenterol 2009;15:2800-4. [PubMed]
- Li X, Law JW, Lee AY. Semaphorin 5A and plexin-B3 regulate human glioma cell motility and morphology through Rac1 and the actin cytoskeleton. Oncogene 2012;31:595-610. [PubMed]
- Li X, Lee AY. Semaphorin 5A and plexin-B3 inhibit human glioma cell motility through RhoGDIalpha-mediated inactivation of Rac1 GTPase. J Biol Chem 2010;285:32436-45. [PubMed]
- Hirota E, Yan L, Tsunoda T, et al. Genome-wide gene expression profiles of clear cell renal cell carcinoma: identification of molecular targets for treatment of renal cell carcinoma. Int J Oncol 2006;29:799-827. [PubMed]
- Dhanabal M, Wu F, Alvarez E, et al. Recombinant semaphorin 6A-1 ectodomain inhibits in vivo growth factor and tumor cell line-induced angiogenesis. Cancer Biol Ther 2005;4:659-68. [PubMed]
- Urbich C, Kaluza D, Fromel T, et al. MicroRNA-27a/b controls endothelial cell repulsion and angiogenesis by targeting semaphorin 6A. Blood 2012;119:1607-16. [PubMed]
- Zhou Q, Gallagher R, Ufret-Vincenty R, et al. Regulation of angiogenesis and choroidal neovascularization by members of microRNA-23~27~24 clusters. Proc Natl Acad Sci U S A 2011;108:8287-92. [PubMed]
- Liu H, Brannon AR, Reddy AR, et al. Identifying mRNA targets of microRNA dysregulated in cancer: with application to clear cell Renal Cell Carcinoma. BMC Syst Biol 2010;4:51. [PubMed]
- Kigel B, Rabinowicz N, Varshavsky A, et al. Plexin-A4 promotes tumor progression and tumor angiogenesis by enhancement of VEGF and bFGF signaling. Blood 2011;118:4285-96. [PubMed]
- Correa RG, Sasahara RM, Bengtson MH, et al. Human semaphorin 6B [(HSA)SEMA6B], a novel human class 6 semaphorin gene: alternative splicing and all-trans-retinoic acid-dependent downregulation in glioblastoma cell lines. Genomics 2001;73:343-8. [PubMed]
- Murad H, Collet P, Huin-Schohn C, et al. Effects of PPAR and RXR ligands in semaphorin 6B gene expression of human MCF-7 breast cancer cells. Int J Oncol 2006;28:977-84. [PubMed]
- Catalano A, Lazzarini R, Di Nuzzo S, et al. The plexin-A1 receptor activates vascular endothelial growth factor-receptor 2 and nuclear factor-kappaB to mediate survival and anchorage-independent growth of malignant mesothelioma cells. Cancer Res 2009;69:1485-93. [PubMed]
- Lazova R, Gould Rothberg BE, Rimm D, et al. The semaphorin 7A receptor Plexin C1 is lost during melanoma metastasis. Am J Dermatopathol 2009;31:177-81. [PubMed]
- Scott GA, McClelland LA, Fricke AF, et al. Plexin C1, a receptor for semaphorin 7a, inactivates cofilin and is a potential tumor suppressor for melanoma progression. J Invest Dermatol 2009;129:954-63. [PubMed]
- Kumanogoh A, Marukawa S, Suzuki K, et al. Class IV semaphorin Sema4A enhances T-cell activation and interacts with Tim-2. Nature 2002;419:629-33. [PubMed]
- Nkyimbeng-Takwi E, Chapoval SP. Biology and function of neuroimmune semaphorins 4A and 4D. Immunol Res 2011;50:10-21. [PubMed]
- Cho WC, Kwan CK, Yau S, et al. The role of inflammation in the pathogenesis of lung cancer. Expert Opin Ther Targets 2011;15:1127-37. [PubMed]
- O’Callaghan DS, O’Donnell D, O’Connell F, et al. The role of inflammation in the pathogenesis of non-small cell lung cancer. J Thorac Oncol 2010;5:2024-36. [PubMed]
- Tauler J, Mulshine JL. Lung cancer and inflammation: interaction of chemokines and hnRNPs. Curr Opin Pharmacol 2009;9:384-8. [PubMed]
- Resende C, Ristimaki A, Machado JC. Genetic and epigenetic alteration in gastric carcinogenesis. Helicobacter 2010;15:34-9. [PubMed]
- Sadanandam A, Rosenbaugh EG, Singh S, et al. Semaphorin 5A promotes angiogenesis by increasing endothelial cell proliferation, migration, and decreasing apoptosis. Microvasc Res 2010;79:1-9. [PubMed]
- Hartwig C, Veske A, Krejcova S, et al. Plexin B3 promotes neurite outgrowth, interacts homophilically, and interacts with Rin. BMC Neurosci 2005;6:53. [PubMed]
- Swiercz JM, Kuner R, Offermanns S. Plexin-B1/RhoGEF-mediated RhoA activation involves the receptor tyrosine kinase ErbB-2. J Cell Biol 2004;165:869-80. [PubMed]
- Artigiani S, Conrotto P, Fazzari P, et al. Plexin-B3 is a functional receptor for semaphorin 5A. EMBO Rep 2004;5:710-4. [PubMed]
- Liu P, Lu Y, Liu H, et al. Genome-wide association and fine mapping of genetic loci predisposing to colon carcinogenesis in mice. Mol Cancer Res 2012;10:66-74. [PubMed]
- Weir BA, Woo MS, Getz G, et al. Characterizing the cancer genome in lung adenocarcinoma. Nature 2007;450:893-8. [PubMed]
- Lu TP, Lai LC, Tsai MH, et al. Integrated analyses of copy number variations and gene expression in lung adenocarcinoma. PLoS One 2011;6:e24829 [PubMed]
- Kettunen E, Salmenkivi K, Vuopala K, et al. Copy number gains on 5p15, 6p11-q11, 7p12, and 8q24 are rare in sputum cells of individuals at high risk of lung cancer. Lung Cancer 2006;54:169-76. [PubMed]
- Bates KE, Whitington PM. Semaphorin 2a secreted by oenocytes signals through plexin B and plexin A to guide sensory axons in the Drosophila embryo. Dev Biol 2007;302:522-35. [PubMed]
- Bishop-Bailey D. PPARs and angiogenesis. Biochem Soc Trans 2011;39:1601-5. [PubMed]
- O'Connor BP, Eun SY, Ye Z, et al. Semaphorin 6D regulates the late phase of CD4+ T cell primary immune responses. Proc Natl Acad Sci U S A 2008;105:13015-20. [PubMed]
- Toyofuku T, Zhang H, Kumanogoh A, et al. Dual roles of Sema6D in cardiac morphogenesis through region-specific association of its receptor, Plexin-A1, with off-track and vascular endothelial growth factor receptor type 2. Genes Dev 2004;18:435-47. [PubMed]
- Zhao XY, Chen L, Xu Q, et al. Expression of semaphorin 6D in gastric carcinoma and its significance. World J Gastroenterol 2006;12:7388-90. [PubMed]
- Dang D, Bamburg JR, Ramos DM. Alphavbeta3 integrin and cofilin modulate K1735 melanoma cell invasion. Exp Cell Res 2006;312:468-77. [PubMed]


