Additional treatment prolonged survival of pulmonary artery sarcoma after surgical resection
Introduction
Pulmonary artery sarcoma (PAS) is an extremely rare malignant tumor, that was first described by Mandelstamm in 1923 (1). Surgical intervention is the mainstay in the treatment for PAS, but the postoperative prognosis remains poor (2). The effect of adjuvant and/or neo-adjuvant chemotherapy and radiotherapy for PAS are controversial (3,4). However, PAS is very rare and there is currently no randomized controlled trial on adjuvant and/or neo-adjuvant chemotherapy for PAS. In this study, to analyze the therapeutic effect of adjuvant therapy on postoperative survival time, we searched MEDLINE and EMBASE database, reviewed a total of 1397 articles published from 1932 to 2018, and selected 162 articles (275 cases).
Methods
Search strategy
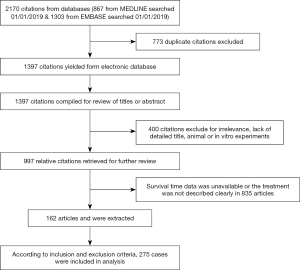
We searched 2 databases, MEDLINE and EMBASE, using keyword “pulmonary artery sarcoma”. Search details were (“pulmonary artery” (MeSH Terms) OR (“pulmonary” (All Fields) AND “artery” (All Fields) OR “pulmonary artery” (All Fields) AND (“sarcoma” (MeSH Terms) OR “sarcoma” (All Fields). The two searched results were combined and duplicated citations were deleted. Two investigators reviewed the abstracts and titles, and excluded citations for irrelevance, lack of detailed title, animal and in vitro experiments. The relative citations were retrieved for further review, and survival time data were extracted. The disagreement was resolved through discussion or arbitration from the third investigator.
Inclusion criteria
- The pathological diagnosis was PAS according to the 2015 World Health Organization classification of tumors.
- Surgery methods included endarterectomy, pneumonectomy, lobectomy, heart-lung transplantation, resection and reconstruction for artery, vessel and heart, except sample or biopsy.
- There was the clear description for therapy strategy based on the surgery, which included chemotherapy, radiotherapy, chemoradiotherapy, target therapy, immunotherapy therapy and so on.
- There were clear clinical records for survival time after initial surgery and second surgery and so on.
- The neoplasm located pulmonary artery, with or without invasion into valve pulmonary artery, right ventricular outflow tract (RVOT), atrium, ventricle.
Exclusion criteria
- Metastatic or secondary malignant tumor.
- Patients refused surgery or accepted surgery just for diagnosis, such as sampling and biopsy.
- Patients died from complications related with surgery during first 30 days.
- Data was incomplete about treatment or survival time after surgery.
Data extraction
Age, gender, postoperative overall survival time, therapeutic approach, tumor extension, tumor localization, status of resection margins and metastasis, surgical method are extracted from abstracts or full texts by our research team.
Statistic method
All statistical analyses were performed using Stata (version MP13.0 for windows Stata Corp LP, USA). Continuous data are expressed as mean ± standard deviation or median and range Median survival was analyzed by Kaplan-Meier method and log-rank test. Surviving patients were censored at the time of last contact. All tests were 2-tailed, and a P value less than 0 .05 was considered statistically significant.
Results
Search and extraction result
A total of 867 citations were searched from MEDLINE and 1,303 citations were searched from EMBASE. The search results were 1,397 citations after removing duplicates. After reading abstracts or titles 400 articles were excluded, because of irrelevance, no detailed title, animal or in vitro experiments. Additional 835 articles were excluded, because patients had not undergone surgery, the survival time data was unavailable or the treatment was not described clearly. In total, 162 articles were extracted. According to inclusion and exclusion criteria, 275 cases were included in analysis (Figure 1).
Patient characteristics
A total of 275 cases were selected including 114 male, 130 female and 31 patients whose gender was unknown. The mean age at diagnosis was 50.9±13.9 years (range: 17 to 86 years old, Table 1). A total of 94 patients underwent only surgical treatment, and 181 patients accepted surgery plus additional treatment (induction chemotherapy, adjuvant chemotherapy, radiation, etc.). There were 141 patients with chemotherapy and 79 patients with radiotherapy. Reconstruction operation was necessary in 118 patients. Ninety patients underwent pulmonary endarterectomy (PEA) and 83 patients received pneumonectomy or lobectomy. Only 10 patients were elected to perform heart-lung transplantation. Twelve patients underwent a second surgical intervention. Usually the combination of operation based on PEA was the preferred procedure according to collected data. A total of 141 patients underwent complete resection (R0), 104 for incomplete resection (R1/2), and the status of the remaining was unknown. There were 100 patients without metastases and 133 with metastases at first surgical treatment. The metastases status was not available for the remaining 42 patients.
Table 1
| Characteristics | No. or mean ± SD |
|---|---|
| Gender | |
| Male | 114 |
| Female | 130 |
| NA | 31 |
| Age (years) | 50.9±13.9 |
| Therapy | |
| (Neo)adjuvant therapy | 181 |
| Surgery only | 94 |
| (Neo)adjuvant therapy | |
| Systemic therapy only | 89 |
| Radiotherapy only | 27 |
| Systemic therapy + radiotherapy | 52 |
| NA | 13 |
| Surgical techniques | |
| PEA | 90 |
| Pneumonectomy or lobectomy | 83 |
| Reconstruction | 118 |
| Transplantation | 10 |
| Second surgery | 12 |
| Location | |
| Pulmonary artery (PA) | 184 |
| Pulmonary trunk (PT) | 151 |
| Pulmonary valve (PV) | 47 |
| Right ventricular outflow tract (RVOT) | 25 |
| Right ventricle (RV) | 5 |
| Right atrium (RA) | 3 |
| Resection status | |
| Incomplete resection (R1/2) | 104 |
| Complete resection (R0) | 141 |
| NA | 30 |
| Metastasis | |
| No | 100 |
| Yes | 133 |
| NA | 42 |
NA, not available.
Survival analyses
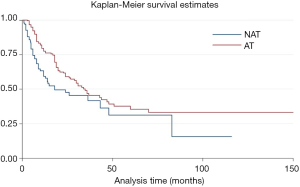
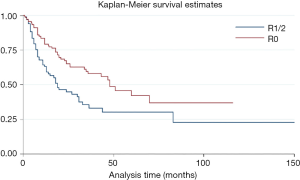
The median postoperative survival time of the entire cohort was 31 months. Patients who received adjuvant and/or neo-adjuvant therapy were associated with improved median survival [hazard ratio (HR) =0.64, P=0.017, 95% confidence interval (CI): 0.45–0.92] (Figure 2).
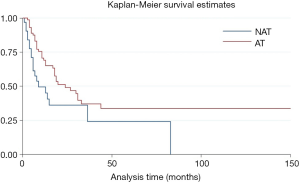
Due to resection margin status the prognosis was different. R0 resection meant longer overall survival time after surgery compared with R1/2 resection (HR =0.55, P=0.002, 95% CI: 0.37–0.79) (Figure S1). For patients with R0 resection, adjuvant and/or neo-adjuvant therapy was seem to have no effect on overall survival time after surgery (HR =0.66, P=0.14, 95% CI: 0.38–1.15). On the other hand, adjuvant and/or neo-adjuvant therapy leaded to a better overall survival time after surgery for patients who underwent a R1/R2 resection (HR =0.53, P=0.025, 95% CI: 0.31–0.92) (Figure 3).
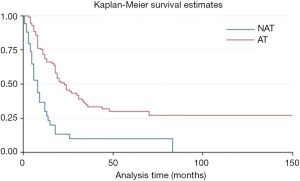
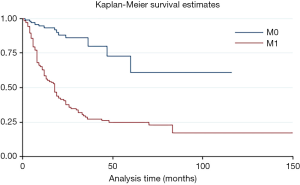
The overall time after surgery was longer for patients without metastasis before or after surgery than with metastasis (HR =6.01, P=0.000, 95% CI: 3.33–10.67) (Figure S2). Moreover, the adjuvant and/or neo-adjuvant therapy lead to a better overall survival time after surgery for patients who had metastasis before or after surgery (HR =0.35, P=0.000, 95% CI: 0.22–0.54) (Figure 4). But those seem to have no effect for patients without metastasis (HR =1.14, P=0.817, 95% CI: 0.37–3.50).
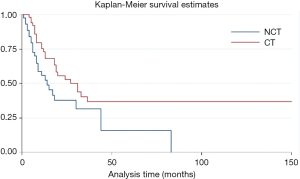
Subgroup analysis suggested that chemotherapy was related with longer overall survival time after surgery (HR =0.63, P=0.015, 95% CI: 0.43–0.91) (Figure 5). Especially, for patients with R1/2 resection (HR =0.53, P=0.026, 95% CI: 0.31–0.93) (Figure S3). But the advantage was not observed in patients with R0 resection (HR =0.79, P=0.43, 95% CI: 0.43–1.44). If the metastasis state was taken into consideration, we found that patients with metastasis, could benefit from chemotherapy (HR =0.44, P=0.000, 95% CI: 0.28–0.68). Otherwise, patients without metastasis could not (HR =0.72, P=0.60, 95% CI: 0.22–2.38).
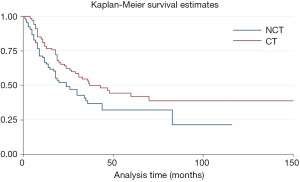
Subgroup analysis also showed that there was no statistical significance between patients with or without radiotherapy (HR =1.04, P=0.83, 95% CI: 0.71–1.53), regardless of resection margin (R0 resection HR =0.97, P=0.92, 95% CI: 0.50–1.85; R1/2 HR =0.79, P=0.424, 95% CI: 0.45–1.40), or metastasis status (M0 HR =0.54, P=0.43, 95% CI: 0.12–2.51; M1 HR =0.82, P=0.37, 95% CI: 0.52–1.31).
Discussion
PAS is often misdiagnosed as acute pulmonary embolism (5), chronic thromboembolic pulmonary hypertension (6), mitral stenosis (7). According to the 2015 World Health Organization classification of tumors, there are several different histologic types of PAS, such as undifferentiated pleomorphic sarcoma, low-grade spindle cell sarcoma with a myxoid background, rhabdomyosarcoma, leiomyosarcoma, undifferentiated sarcoma with epithelioid or round cell features, synovial sarcoma, epithelioid hemangioendothelioma, and angiosarcoma (8). Surgical resection is the primary treatment and greatly prolongs the overall survival time (9). A few patients have long-term survival after radical resection (10-13). Depending on tumor localization, local invasion, and distal metastases, surgical methods include extensive mass resection, endarterectomy, reconstruction of the great vessels or heart (14), lobectomy (15), pneumonectomy (10), and combined lung-heart transplantation (16). The combination of different surgical methods is needed in some patients requiring complete resection or satisfied tumor cytoreduction (17). Our analysis has shown that complete resection can decrease the possibility of metastasis (Table 2). Moreover, studies have suggested that aggressive surgical resection may increase the postoperative survival time in patients with PAS (18).
Table 2
| Therapy | R0 | R1/2 | M1 | M0 |
|---|---|---|---|---|
| (Neo)adjuvant therapy | 87 | 72 | 98 | 59 |
| No (neo)adjuvant therapy | 54 | 30 | 33 | 41 |
| Chemotherapy | 73 | 50 | 79 | 47 |
| No chemotherapy | 53 | 43 | 46 | 48 |
| Radiotherapy | 36 | 34 | 46 | 24 |
| No radiotherapy | 89 | 59 | 79 | 70 |
Endarterectomy has been widely used in operations since 1990’s (19), despite as a palliative resection, because it may be suitable to the neoplasm located in the pulmonary trunk, pulmonary artery and proximal part of pulmonary lobar artery. Due to misdiagnosis as pulmonary embolism, PEA is frequently taken in emergency surgery (20), and the technical details of PEA are similar to chronic thromboembolism pulmonary hypertension (CTEPH) (21). Except that neoplasm is located in only distal parts of pulmonary artery, lobectomy or pneumonectomy is usually performed as additional surgery of endarterectomy (22,23). A few researchers report that the patients, who are underwent lobectomy or pneumonectomy (2), especially pneumonectomy (10,11), may have longer overall survival time, although the efficacy may be affected by tumor localization and lung parenchymal involvement.
The reconstruction of the great vessel or heart is a complex procedure for the radical resection. The reconstruction strategy includes pulmonary artery reconstruction (24), reconstruction of pulmonary trunk (25), pulmonary valve replacement (26), ventriculotomy and reconstruction (13,18), which is determined on preoperative and intraoperative assessment by surgeons. The combined lung-heart transplantation is an aggressive surgical method. Talbot et al. reported 2 cases, who underwent combined heart-lung transplantation for unresectable primary pulmonary artery sarcoma. Both patients were given chemotherapy before transplantation (16). The overall survival time (49 and 48 months, respectively) after transplantation is remarkable. Nevertheless, the cases are too rare to evaluate the prognosis.
Metastases are most commonly found in the lungs, although cases in the pancreas, kidney, brain, lymph nodes, thyroid, adrenal glands and skin have been reported (27,28). Appropriately half of cases have developed metastases before death, and the prognosis of these patients appears to be quite poor. The median of overall survival time after surgery is 19.5 months in patients without metastasis and 13 months in patients with metastasis. The main treatment of choice is chemotherapy, but multiple repeated metastasectomy or radiotherapy had been performed in some patients (28,29). For patients with unstable hemodynamics, palliative interventional (pulmonary artery) PA stenting performed under local anesthesia can improve the quality of life by reducing excessive pulmonary hypertension in a short time (30-32). The knowledge on the benefit of adjuvant and/or neo-adjuvant adjuvant therapy is limited. Some researchers believe that chemotherapy or radiotherapy has no effect on long-term survival (33), whereas others suggest that it can prolong survival. Mussot et al. reported 31 patients who were surgically treated for pulmonary artery sarcoma. Six patients had neoadjuvant chemotherapy and 18 patients received adjuvant therapy including 15 for chemotherapy, 2 for radiotherapy and only 1 for chemoradiotherapy. There was no significant difference in the survival between the two groups with or without (neo) adjuvant therapy (34).
Lee and colleagues (35) collected 20 patients. The median overall survival for all patients was 24 months. They used Cox proportional hazard model analysis to identify factors influencing mortality and found that chemotherapy was negatively associated with mortality (HR =0.102, P=0.032, 95% CI: 0.013–0.826) Similar findings were reported by Blackmon (36) and Wong (3). In our analysis, patients are divided into adjuvant and/or neo-adjuvant therapy group if they have the history of adjuvant and/or neo-adjuvant therapy including chemotherapy, radiotherapy, chemoradiotherapy, target therapy, immunotherapy and anti-angiogenesis drug. Compared to patients with only surgery, adjuvant and/or neo-adjuvant therapy can improve the prognosis of patients who have surgical treatment, statistical analysis is made briefly based on chemotherapy and/or radiotherapy, because of the limited number of other therapies. Furthermore, the patients benefit from chemotherapy obviously, especially with metastasis or incomplete resection. On the other hand, if patients have complete resection and no metastasis, there is no statistical significance on the overall survival time after surgery whether patients receive chemotherapy, But the median of overall survival time after surgery in the chemotherapy group is longer, regardless of metastasis or resection status. Less than half of patients underwent complete resection because of surgical complexity, and more than half of patients developed metastasis before death. Nevertheless, patients with complete resection still have a high risk for metastasis, so most patients need chemotherapy after surgery except bad performance status or refusal. Although unanimous recommendation can’t be reached, common chemotherapy regimens are taken as follows (Table 3). Two-drug regimen is preferred as first-line therapy in a majority of cases (23,40-44), one-drug or three-drug regimen is alternative second-line therapy or maintenance (3,38,49).
Table 3
| Chemotherapy regimens | Drug name(s) |
|---|---|
| One-drug | Doxorubicin (37)★, ifosfamide (38,39) |
| Two-drug | Ifosfamide + epirubicin (40,41) |
| Ifosfamide + doxorubicin (23,42-44)★ | |
| Cisplatin + etoposide (45) | |
| Carboplatin + amrubicin (46)★ | |
| Carboplatin + vinorelbine (47) | |
| Gemcitabine + docetaxel (48)★ | |
| Three-drug | Vinorelbine + cisplatin + cyclophosphamide (38) |
| Vincristine + doxorubicin + cyclophosphamide (38) | |
| Doxorubicin + ifosfamide + dacarbazine (49)★ | |
| Epirubicin + doxorubicin + ifosfamide (3) | |
| Ifosfamide + doxorubicin + mesna (50,51) |
★, patients present rapid partial response (PR) shortly after chemotherapy.
Survival analysis shows that radiotherapy can’t improve the survival time regardless of metastasis or incisal margin status. About one third patients received radiotherapy in our data. However, radiotherapy is still suggested in case of incomplete surgery (20,52,53), local recurrence (21) or distal metastasis (47). The purpose and timing of radiotherapy was not described in most articles, so its effect on local recurrence and distal metastasis is difficult to analyze. The radiological dose of (computed tomography venography) CTV is 4,500–6,600 cGy (13,20,28,54,55) in conventional fraction, and some metastasis patients received stereotactic body radiation therapy (12 Gy ×5 fractions) (56). Yin et al. (22) showed that patients who received postoperative combined chemo- and radiotherapy is associated with improved outcomes (median survival 28 vs. 8 months, P=0.042). But in our series, combination of chemotherapy and radiotherapy neither prolonged survival time nor decreased mortality.
Limitation
Our statistical analysis is made on the basis of few available data because PAS is so rare that there is no randomized controlled trial or cohort study. PAS is mostly presented in case report or a series of case reports in the literature. Moreover, full text can’t be acquired for some of the citations. Some citations did not clearly describe the survival time after surgery and treatment strategy, and complete data can’t be extracted in these cases. On the other hand, it’s difficult to reduce the publication bias and selection bias. Some patients with long-term survival time stand out in case series, although it has no remarkable influence on the statistic results.
Conclusions
In summary, PAS is an extremely rare malignancy with a very poor prognosis. While radical surgery offers the only chance of cure, palliative and aggressive surgery can relieve the symptoms and extend the survival. Patients may benefit from adjuvant and/or neo-adjuvant chemotherapy, especially in case of metastasis or incomplete resection.
Acknowledgments
Funding: This study was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.02.80). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mandelstamm M. Über primäre Neubildungen des Herzens. Virchows Archiv für pathologische Anatomie und Physiologie und für klinische Medizin 1923;245:43-54. [Crossref]
- Grazioli V, Vistarini N, Morsolini M, et al. Surgical treatment of primary pulmonary artery sarcoma. J Thorac Cardiovasc Surg 2014;148:113-8. [Crossref] [PubMed]
- Wong HH, Gounaris I, McCormack A, et al. Presentation and management of pulmonary artery sarcoma. Clin Sarcoma Res 2015;5:3. [Crossref] [PubMed]
- Bakaeen FG, Jaroszewski D, Rice D, et al. Outcomes after surgical resection of cardiac sarcoma in the multimodality treatment era. J Thorac Cardiovasc Surg 2009;137:1454-60. [Crossref] [PubMed]
- Jiang S, Li J, Zeng Q, et al. Pulmonary artery intimal sarcoma misdiagnosed as pulmonary embolism: A case report. Oncol Lett 2017;13:2713-6. [Crossref] [PubMed]
- Wilkens H, Konstantinides S, Lang I, et al. Chronic thromboembolic pulmonary hypertension: Recommendations of the Cologne Consensus Conference 2016. Dtsch Med Wochenschr 2016;141:S62-9. [PubMed]
- Spartalis M, Tzatzaki E, Spartalis E, et al. Primary cardiac intimal sarcoma masquerading as mitral stenosis. Clin Case Rep 2017;5:1422-3. [Crossref] [PubMed]
- Travis WD, Brambilla E, Burke AP, et al. Introduction to The 2015 World Health Organization Classification of Tumors of the Lung, Pleura, Thymus, and Heart. J Thorac Oncol 2015;10:1240-2. [Crossref] [PubMed]
- Scheidl S, Taghavi S, Reiter U, et al. Intimal sarcoma of the pulmonary valve. Ann Thorac Surg 2010;89:e25-7. [Crossref] [PubMed]
- Stella F, Davoli F, Brandolini J, et al. Pulmonary artery leiomyosarcoma successfully treated by right pneumonectomy. Asian Cardiovasc Thorac Ann 2009;17:513-5. [Crossref] [PubMed]
- Linden PA, Morgan JA, Couper GS. Seven-year disease-free survival after radical pneumonectomy for a pulmonary artery sarcoma. J Thorac Cardiovasc Surg 2013;146:e17-8. [Crossref] [PubMed]
- Chaubey S, Benson C, Khan H, et al. Six-year survival of a patient with pulmonary artery angiosarcoma. Asian Cardiovasc Thorac Ann 2012;20:728-30. [Crossref] [PubMed]
- Head HD, Flam MS, John MJ, et al. Long-term palliation of pulmonary artery sarcoma by radical excision and adjuvant therapy. Ann Thorac Surg 1992;53:332-4. [Crossref] [PubMed]
- Hou Y, Shen Z, Gao W, et al. Pulmonary artery intimal sarcoma: case report. J Card Surg 2010;25:29-31. [Crossref] [PubMed]
- Shen W, Chen J, Wei S, et al. Primary pulmonary leiomyosarcoma. J Chin Med Assoc 2014;77:49-51. [Crossref] [PubMed]
- Talbot SM, Taub R, Keohan M, et al. Combined heart and lung transplantation for unresectable primary cardiac sarcoma. J Thorac Cardiovasc Surg 2002;124:1145-8. [Crossref] [PubMed]
- Zerkowski HR, Hofmann H, Gybels I, et al. Primary sarcoma of pulmonary artery and valve: multimodality treatment by chemotherapy and homograft replacement. J Thorac Cardiovasc Surg 1996;112:1122-4. [Crossref] [PubMed]
- Yamamoto Y, Shintani Y, Funaki S, et al. Aggressive Surgical Resection of Pulmonary Artery Intimal Sarcoma. Ann Thorac Surg 2018;106:e197-9. [Crossref] [PubMed]
- Redmond ML, Shepard JW Jr, Gaffey TA, et al. Primary pulmonary artery sarcoma. A method of resection. Chest 1990;98:752-3. [Crossref] [PubMed]
- Sakai K, Minoura Y, Matsui T, et al. Primary Pulmonary Artery Intimal Sarcoma Case with Elevated Coagulation Markers. J Clin Diagn Res 2017;11:OD10-1. [PubMed]
- Deng L, Zhu J, Xu J, et al. Clinical presentation and surgical treatment of primary pulmonary artery sarcoma. Interact Cardiovasc Thorac Surg 2018;26:243-7. [Crossref] [PubMed]
- Yin K, Zhang Z, Luo R, et al. Clinical features and surgical outcomes of pulmonary artery sarcoma. J Thorac Cardiovasc Surg 2018;155:1109-15.e1. [Crossref] [PubMed]
- Morreau SP, Haydock DA. Prolonged Survival of Pulmonary Artery Sarcoma After Aggressive Surgical Resection. Ann Thorac Surg 2017;103:e21-3. [Crossref] [PubMed]
- Obeso Carillo GA, Casais Pampin R, Legarra Calderon JJ, et al. Primary pulmonary artery sarcoma: a new surgical technique for pulmonary artery reconstruction using a self-made stapled bovine pericardial graft conduit. Eur J Cardiothorac Surg 2015;47:188-90. [Crossref] [PubMed]
- Mei J, Pu Q, Zhu Y, et al. Reconstruction of the pulmonary trunk via cardiopulmonary bypass in extended resection of locally advanced lung malignancies. J Surg Oncol 2012;106:311-5. [Crossref] [PubMed]
- Jelacic S, Meguid RA, Oxorn DC. Near total occlusion of the main pulmonary artery and destruction of pulmonary valve by leiomyosarcoma. Anesth Analg 2013;116:53-6. [Crossref] [PubMed]
- Rashid A, Molloy S, Lehovsky J, et al. Metastatic pulmonary intimal sarcoma presenting as cauda equina syndrome: first report of a case. Spine 2008;33:E516-20. [Crossref] [PubMed]
- Choi YM, Jang EK, Ahn SH, et al. Long-term survival of a patient with pulmonary artery intimal sarcoma after sequential metastasectomies of the thyroid and adrenal glands. Endocrinol Metab (Seoul) 2013;28:46-9. [Crossref] [PubMed]
- Said SM, Sundt TM 3rd, Garces YI, et al. 5-year survival after multiple repeat metastasectomy for pulmonary artery angiosarcoma. Ann Thorac Surg 2011;91:e49-51. [Crossref] [PubMed]
- Meckel S, Buitrago-Téllez C, Herrmann R, et al. Stenting for pulmonary artery stenosis due to a recurrent primary leiomyosarcoma. J Endovasc Ther 2003;10:141-6. [Crossref] [PubMed]
- Mendiz O, Lev G, Valdivieso L, et al. Lifesaving kissing stent for pulmonary trunk stenosis due to primary angiosarcoma. Ann Vasc Surg 2010;24:1135.e9-12. [Crossref] [PubMed]
- Kissling P, Brosi P, Kull C, et al. Implantation of a stent graft in the right pulmonary artery enables radical resection of a central endothelial sarcoma of the left pulmonary artery. J Vasc Surg 2013;58:787-9. [Crossref] [PubMed]
- Anderson MB, Kriett JM, Kapelanski DP, et al. Primary pulmonary artery sarcoma: a report of six cases. Ann Thorac Surg 1995;59:1487-90. [Crossref] [PubMed]
- Mussot S, Ghigna MR, Mercier O, et al. Retrospective institutional study of 31 patients treated for pulmonary artery sarcoma. Eur J Cardiothorac Surg 2013;43:787-93. [Crossref] [PubMed]
- Lee Y, Kim HJ. Clinical Characteristics and Treatment Outcomes of Primary Pulmonary Artery Sarcoma in Korea. 2016;31:1755-60.
- Blackmon SH, Rice D, Correa A, et al. Management of primary pulmonary artery sarcomas. Ann Thorac Surg 2009;87:977-84. [Crossref] [PubMed]
- Arai J, Yamasaki N, Miyazaki T, et al. Leiomyosarcoma of pulmonary artery origin diagnosed preoperatively. Kyobu Geka 2012;65:461-5. [PubMed]
- Xu Y, Wang K, Geng Y, et al. A case of intimal sarcoma of the pulmonary artery successfully treated with chemotherapy. Int J Clin Oncol 2012;17:522-7. [Crossref] [PubMed]
- Maekura T, Shimizu S, Kawaguchi T, et al. Intravascular synovial sarcoma of the pulmonary artery with massive pleural effusion: report of a case with a favorable response to Ifosfamide chemotherapy and palliative radiation therapy. Intern Med 2015;54:1095-8. [Crossref] [PubMed]
- Uchida A, Tabata M, Kiura K, et al. Successful treatment of pulmonary artery sarcoma by a two-drug combination chemotherapy consisting of ifosfamide and epirubicin. Jpn J Clin Oncol 2005;35:417-9. [Crossref] [PubMed]
- Ozbek C, Emrecan B, Calli A, et al. Intimal sarcoma of the pulmonary artery with retrograde extension into the pulmonic valve and right ventricle. Tex Heart Inst J 2007;34:119-21. [PubMed]
- Austin BA, Griffin B. Pulmonary artery intimal sarcoma: a brief case series. J Am Soc Echocardiogr 2008;21:978.e5-7. [Crossref] [PubMed]
- Hsing JM, Thakkar SG, Borden EC, et al. Intimal pulmonary artery sarcoma presenting as dyspnea: case report. Int Semin Surg Oncol 2007;4:14. [Crossref] [PubMed]
- Schreiner M, Sanad W, Pfitzner BM, et al. A primary intravascular synovial sarcoma causing deep-vein thrombosis and pulmonary embolism in a 20-year-old woman. Curr Oncol 2015;22:e387-90. [Crossref] [PubMed]
- Li B, Zhang Y, Cai L, et al. Primary pulmonary artery sarcoma differentiated from pulmonary thromboembolism by ventilation-perfusion scan. Long survival of the patient. Hell J Nucl Med 2015;18:166-8. [PubMed]
- Hirose T, Ishikawa N, Hamada K, et al. A case of intimal sarcoma of the pulmonary artery treated with chemoradiotherapy. Intern Med 2009;48:245-9. [Crossref] [PubMed]
- Cantaloube M, Moureau-Zabotto L, Mescam L, et al. Metastatic Intimal Sarcoma of the Pulmonary Artery Sensitive to Carboplatin-Vinorelbine Chemotherapy: Case Report and Literature Review. Case Rep Oncol 2018;11:21-8. [Crossref] [PubMed]
- Austin BA, Griffin BP. Pulmonary artery intimal sarcoma: a brief case series. J Am Soc Echocardiogr 2008;21:978.e5-7. [Crossref] [PubMed]
- Muturi A, Kotecha V, Ruturi J, et al. High-grade spindle cell sarcoma of the heart: a case report and review of literature. J Cardiothorac Surg 2015;10:46. [Crossref] [PubMed]
- Penel N, Taieb S, Ceugnart L, et al. Report of eight recent cases of locally advanced primary pulmonary artery sarcomas: failure of Doxorubicin-based chemotherapy. J Thorac Oncol 2008;3:907-11. [Crossref] [PubMed]
- Chan SH, Tsai LM, Tsai WC, et al. Pulmonary artery leiomyosarcoma. J Formos Med Assoc 1999;98:578-81. [PubMed]
- Choong CK, Lawton J, Moon M, et al. Failure of medical therapy for pulmonary "thromboembolic" disease: beware the unsuspected primary sarcoma of the pulmonary artery. J Thorac Cardiovasc Surg 2004;128:763-5. [Crossref] [PubMed]
- Cantaloube M, Moureau-Zabotto L, Mescam L, et al. Metastatic Intimal Sarcoma of the Pulmonary Artery Sensitive to Carboplatin-Vinorelbine Chemotherapy: Case Report and Literature Review. Case Rep Oncol 2018;11:21-8. [Crossref] [PubMed]
- Faul JL, Wahl T, Ihnken K, et al. Superior vena cava syndrome caused by pulmonary artery sarcoma. J Thorac Cardiovasc Surg 1999;118:749-50. [Crossref] [PubMed]
- Hoogma D, Meyns B, Van Raemdonck D, et al. Anesthetic Management for Resection of Bilateral Pulmonary Artery Sarcoma. A A Case Rep 2015;5:64-8. [Crossref] [PubMed]
- D'Armini A M, Garcia-Cabezas S, Centeno-Haro M, et al. Intimal sarcoma of the pulmonary artery with multiple lung metastases: Long-term survival case. World J Clin Oncol 2017;8:366-70. [Crossref] [PubMed]