Sclerosing thymoma: a case report and literature review
Introduction
Sclerosing thymoma is a rare thymoma subtype with exuberant collagen-rich stroma. Since Dr. T KUO named the first case in 1994 (1), all the lesions in 16 described cases on PUBMED (1-6) seemed to behave benignly (6,7). Here, we highlighted an invasive one, which pertains a differential diagnosis dilemma of other malignancies such as NSHL (Nodular sclerosis Hodgkin’s lymphoma), etc. with genetics features manifested by NGS (next-generation sequencing) data. We presented the following case in accordance with the CARE Guideline (1-6).
Case presentation
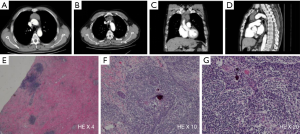
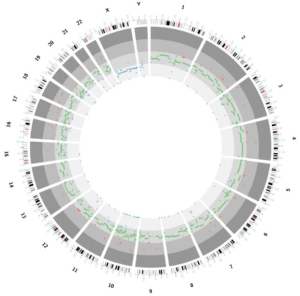
A 53-year-old male underwent a CT scan for mild dyspnea concerning pneumonia. CT imaging revealed a 4.3×2.5 cm soft tissue mass in the upper anterior mediastinum (Figure 1A). The heterogeneous attenuated tumor assumed an irregular shape, extended into the mediastinal fat and became indistinguishable from the pericardium and left brachiocephalic vein (Figure 1B,C,D). Mild non-uniform enhancement (CT value ranged 26–56 Hu) appeared on the arterial phase imaging. No regional lymphadenopathy was seen. The patient had a surgical history of varicose veins in the lower extremities 5 years ago and had no other underlying diseases. He had been a painter and had a history of contact with paint. He has been smoking cigarettes for more than 30 years, with a smoking index of 15 pack-years. His father has a history of lung cancer. The surgeon cautiously separated the adhesions and struggled to complete removal of the tumor. Under the microscope, thymoma-like epithelial cell islands scattered in a fibrotic background with interstitial hyaline degeneration, calcification, and multinuclear giant cell reaction. Glandular epithelial cells deposit in a small part (Figure 1E,F,G). The lesion boundary was unclear, invading the surrounding fat and the left brachiocephalic vein. Pathological stage was T3 according to AJCC/UICC TNM staging system, and stage III based on Masaoka-Koga classification. Though the surgical margin of the patient was negative, the tumor invaded the surrounding fat and left brachiocephalic vein. There was a risk of recurrence of the local advanced tumor. Whole-exome sequencing showed no aberrations of related signaling pathways. Copy-number variation analysis indicated deletions in chromosomes 2/3/7/8/10/12/22/22/X as well as amplifications in chromosomes 1/2/3/7/9/10/16/17/18 (Figure 2). For the locally advanced disease, radiation was delivered to the tumor bed at a dose of 95% PTV 50 Gy with conventional fractionation. CT evaluation of chest and abdomen was performed 1 month after radiotherapy, followed by revisit 3 months later. After 2 years’ regular follow-up, the patient showed no special discomfort and no recurrence or metastasis was found (Figure 3).
Discussion
So far, only 16 cases can be found from literature (Table 1). We presented here the first invasive one with genetics features. In all, the median age of onset was 57.5 years (range, 23–78 years). The incidences in males and females were roughly similar. Among the published sixteen cases, half were asymptomatic at diagnosis. Three manifested myasthenia gravis. Others complained of mild chest tightness or shortness of breath associated with a larger tumor size. The documented tumors formed well-circumscribed masses with an average diameter of 5 cm (range, 2 to 10 cm). Cut surfaces were homogeneously light tan, firm to hard in areas of extensive sclerosis and calcification. No hemorrhage or necrosis was seen (8). Microscopically, 85% to 90% of the tumor was characteristic hyalinized fibroconnective tissue (6). In seven tumors, cellular aggregates comprised double populations of epithelial cells and lymphocytes. In another three, those aggregates featured spindle cells with scant eosinophilic cytoplasm. No cellular atypia or mitosis was observed. Cellular aggregates were embedded haphazardly in the extensive areas of collagenization. In one case, the proliferated cells dissected the fibrocollagen, mimicking a vascular neoplasia. Epithelial cells scattered in a myxoid-appearing background in focal areas with dense collagenization.
Table 1
| Author | Sex/age | Clinical manifestations | Tumor size (cm) | Follow-up outcomes |
|---|---|---|---|---|
| Kuo 1994 (1) | F/39 | Myasthenia gravis | 3 | Alive at 4-year |
| F/23 | Myasthenia gravis | 2.5 | Alive at 2-year | |
| Moran 2004 (6) | F/34 | Asymptomatic | 5 | Alive at 1 year |
| M/58 | Asymptomatic | 6 | Died, congestive heart failure | |
| M/44 | Asymptomatic | 5 | Lost to follow-up | |
| M/56 | Asymptomatic | 10 | Lost to follow-up | |
| F/62 | Asymptomatic | 8 | Alive at 6-year | |
| F/37 | Shortness of breath, chest pain | 6 | Died, pulmonary edema | |
| M/69 | Shortness of breath, chest pain | 7 | Died, renal insufficiency | |
| M/59 | Shortness of breath, chest pain | 6 | Died, congestive heart failure | |
| F/27 | Myasthenia gravis | 5 | Died, cause unknown | |
| M/73 | Shortness of breath, chest pain | 10 | Died, cause unknown | |
| Kim 2006 (5) | M/47 | Asymptomatic | 2 | Lost to follow-up |
| Tajima 2015 (4) | F/62 | Asymptomatic | 3.1 | Lost to follow-up |
| Kato 2017 (3) | F/78 | General malaise | 7.8 | Alive at 3 years |
| Li 2008 (2) | M/65 | Asymptomatic | 4.9 | Alive at 2 years |
Thymoma accounts for 20% of mediastinal tumors and 50% of anterior mediastinal masses in adults (9). Sclerosing thymoma, the rare subtype, represents <1% (6) Its etiology remains a mystery. A hyalinized, fibrosclerotic stroma dominates the tumor mass that expands into septa, perivascular spaces, and the tumor periphery (8). This profuse stromal expansion was perceived as either stimulated by the neoplastic thymic epithelium or regressive changes. Summoned Congo red staining might foreground the embedded strands of neoplastic epithelial cells barely detectable on H&E staining (3). Notably, a fibrous mass within thymus must be thoroughly sampled to avoid missing microscopic thymoma lesion. Immunohistochemical staining for keratin (AE1/AE3) may highlight the epithelial component in these tumors, and the neuroendocrine markers (chromogranin A, synaptophysin, CD56) were negative (10). Dystrophic calcification, cholesterol granulomas, and small cysts are commonly seen (1,5). The tumor may lack immature T cells. No specific pathological biomarkers or molecular changes in genes have surfaced for diagnosing sclerosing thymoma.
One vital issue is whether these tumors are invasive or noninvasive. Discrimination is difficult for areas diagnosed as thymomas are always within extensive sclerosis or hyalinization, leading to the interpretation that these lesions are probably encapsulated, and more likely to be diagnosed as non-invasive thymomas. One possible explanation for this phenomenon might not necessarily be related to a regression change but rather to “ancient” changes in these tumors (1,6). These tumors might linger long enough for the characteristic “fibrocollagenous bands” in thymomas, to coalesce around the residual focal cellular components. Serial sectioning of such a lesion avoids failing to catch a microscopic invasion.
Sclerosing thymoma may spontaneously regress regardless of the histological type. Spontaneous regression of malignancies is mediated by two biological pathways: differentiation of the malignant cells to a normal phenotype, or cell death through apoptosis or inflammatory necrosis. The mechanism of the spontaneous regression in sclerosing thymoma is still unknown since the residual thymomas were found only after serial sectioning. The author suggested that a fibrous mass in thymus has to be thoroughly sampled to avoid missing a microscopic thymoma (1).
Sclerosing thymoma is susceptible to alternative diagnoses within the mediastinum. Its prominent hyalinization and sclerosis make the neoplastic areas inconspicuous. Sclerosing mediastinitis forms irregular solid masses composed of fibrosclerotic tissue with interspersed inflammatory infiltrates and, at best, minimal remnant thymic epithelial cells. Sclerosing mediastinitis features hyalinized fibrocollagen with only foci of inflammatory aggregates rather than the dual cell population in thymomas. Also, sclerosing mediastinitis lacks focal areas of spindle epithelial cells. Solitary fibrous tumor is a spindle cell tumor with collagenous stroma. Hypocellular and hypercellular areas coexist, displaying a hemangiopericytic pattern or patterns associated with other types of sarcomas. Immunophenotype is positive for CD34, CD99, Bcl2, and STAT6, while negative for keratin-positive epithelial cells. The main sclerotic lymphomas are classical Hodgkin lymphoma and primary mediastinal large B-cell lymphoma (8). In cases of lymphoma with prominent sclerosis, the tumor will show areas of immature lymphoid components rather than the biphasic differentiation in thymic sarcomas, including desmoplastic small round cell tumor (11). Differential diagnoses include germ cell tumors, metastases with desmoplastic stroma, fungal infections, granulomatous vasculitis, and IgG4-related diseases. Among all these alternatives, the diagnosis cannot be reached if the only areas sampled correspond to hyalinized material. In this setting, one needs to include thymomas among the possible considerations in the differential diagnosis.
For sclerosing thymoma, complete surgical removal seems appropriate. Those who cannot achieve complete resection should consider radiotherapy, either external beam radiation, brachytherapy or intra-operative radiation. Our case is the first invasive case requiring postoperative adjuvant radiotherapy. Follow-up results show no evidence of local recurrence or distant metastasis. No proposed chemotherapy regimen or targeted therapy is available. In a case of sclerosing thymoma-like thymic amyloidoma reported in 2017 (3), a 78-year-old female with Masaoka IVa thymoma received pulse steroids for paraneoplastic nephrotic syndrome (minimal change disease). A marked reduction in tumor size was observed on maintenance therapy with prednisolone. Tumor regression can be induced by an increased steroid level. The author called for further study in the efficacy of steroid therapy for sclerosing thymoma.
The prognosis of thymoma is mainly related to the Masaoka staging, WHO histological subtypes. Other clinicopathological factors, such as complete resection, pleural involvement, and tumor size may also account. Disregarding histology and staging, all types of thymoma can invade and metastasize, i.e., potentially malignant. The prognostic profile of sclerosing thymoma needs more observation to assess.
Sclerosing thymoma, previously considered noninvasive, could also be invasive as our case is. Scrutinizing the cellular components against a background of abundant collagenization is key to its differentiation from other mediastinal diseases. Better understanding of the potential malignancy and long-term follow-up of this thymoma subtype will pave the road for its optimal management.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.02.40). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kuo T. Sclerosing thymoma--a possible phenomenon of regression. Histopathology 1994;25:289-91. [Crossref] [PubMed]
- Li X, Wang M, Sun D. Sclerosing thymoma: A rare case report and brief review of literature. Medicine (Baltimore) 2018;97:e0520. [Crossref] [PubMed]
- Kato Y, Okuda M, Fukuda K, et al. Sclerosing thymoma-like thymic amyloidoma with nephrotic syndrome: a case report. J Med Case Rep 2017;11:216. [Crossref] [PubMed]
- Tajima S, Koda K. A case report of sclerosing thymoma of the anterior mediastinum: an exceedingly rare morphology. Int J Clin Exp Pathol 2015;8:4233-7. [PubMed]
- Kim YH, Ishii G, Naito Y, et al. A resected case of sclerosing thymoma. Nihon Kokyuki Gakkai Zasshi 2006;44:420-3. [PubMed]
- Moran CA, Suster S. "Ancient" (sclerosing) thymomas: a clinicopathologic study of 10 cases. Am J Clin Pathol 2004;121:867-71. [Crossref] [PubMed]
- Chatterjee D, Finch CJ. Sclerosing Thymoma. Arch Pathol Lab Med 2015;139:1068-70. [Crossref] [PubMed]
- WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. WHO/IARC Classification of Tumours, 4th Edition 2015;7.
- Strollo DC, Rosado de Christenson ML, Jett JR. Primary mediastinal tumors. Part 1: tumors of the anterior mediastinum. Chest 1997;112:511-22. [Crossref] [PubMed]
- Yutaka Y, Omasa M, Shikuma K, et al. Spontaneous regression of an invasive thymoma. Gen Thorac Cardiovasc Surg 2009;57:272-4. [Crossref] [PubMed]
- Nayak HK, Vangipuram DR, Sonika U, et al. Mediastinal mass-a rare presentation of desmoplastic small round cell tumour. BMJ Case Rep 2011; [Crossref] [PubMed]